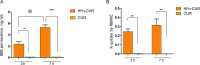Exploring the anti-inflammatory effects of curcumin encapsulated within ferritin nanocages: a comprehensive in vivo and in vitro study in Alzheimer's disease
- PMID: 39551771
- PMCID: PMC11571668
- DOI: 10.1186/s12951-024-02897-4
Exploring the anti-inflammatory effects of curcumin encapsulated within ferritin nanocages: a comprehensive in vivo and in vitro study in Alzheimer's disease
Abstract
Background: The global demographic shift towards an aging population is generating a rise in neurodegenerative conditions, with Alzheimer's disease (AD) as the most prominent problem. In this landscape, the use of natural supplements has garnered attention for their potential in dementia prevention. Curcumin (Cur), derived from Curcuma longa, has demonstrated promising pharmacological effects against AD by reducing the levels of inflammatory mediators. However, its clinical efficacy is hindered by poor solubility and bioavailability. Our study introduces the use of H-Ferritin nanocages (HFn) as a nanoformulation vehicle for Cur, aiming to enhance its therapeutic potential for AD. In this work, we characterized a nanoformulation of Cur in HFn (HFn-CUR) by evaluating its safety, stability, and its transport across the blood-brain barrier (BBB) in vitro. Moreover, we evaluated the efficacy of HFn-CUR by transcriptomic analysis of peripheral blood mononuclear cells (PBMCs) from both AD patients and healthy controls (HC), and by using the well-established 5xFAD mouse model of AD.
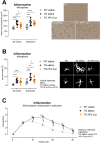
Results: Our data show that HFn-CUR exhibits improved water dispersibility, is non-toxic, and can traverse the BBB. Regarding its activity on PBMCs from AD patients, HFn-CUR enhances cellular responses to inflammation and reduces RAGE-mediated stress. Studies on an AD mouse model demonstrate that HFn-CUR exhibits mild beneficial effects on cognitive performance. Moreover, it effectively reduces microgliosis and astrogliosis and in vivo in mouse, suggesting potential neuroprotective benefits.
Conclusions: Our data suggest that HFn-CUR is safe and effective in reducing inflammation in both in vitro and in vivo models of AD, supporting the need for further experiments to define its optimal use.
Keywords: 5xFAD mice; Alzheimer’s disease; Blood-brain barrier; Curcumin; H-Ferritin; Nanoparticles; Neuroinflammation; Toxicity.
© 2024. The Author(s).
Conflict of interest statement
Figures