Phase 1 clinical trial of Hantaan and Puumala virus DNA vaccines delivered by needle-free injection
- PMID: 39551791
- PMCID: PMC11570633
- DOI: 10.1038/s41541-024-00998-7
Phase 1 clinical trial of Hantaan and Puumala virus DNA vaccines delivered by needle-free injection
Abstract
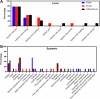
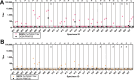
Hantaan virus (HTNV) and Puumala virus (PUUV) are pathogenic zoonoses found in Asia and Europe, respectively. We conducted a randomized Phase 1 clinical trial of individual HTNV and PUUV DNA vaccines targeting the envelope glycoproteins (GnGc), as well as a combined HTNV/PUUV DNA vaccine delivered at varying doses using the PharmaJet Stratis® needle-free injection system (NCT02776761). Cohort 1 and 2 vaccines consisted of 2 mg/vaccination of HTNV or PUUV plasmid, respectively. Cohort 3 vaccine consisted of 2 mg/vaccination of 1:1 mixture of HTNV and PUUV vaccines. Vaccinations were administered on Days 0, 28, 56, and 168. The vaccines were safe and well tolerated. Neutralizing antibody responses were elicited in 7/7 (100%) subjects who received the HTNV DNA (Cohort 1) and 6/6 (100%) subjects who received the PUUV DNA (Cohort 2) vaccines alone. The combination vaccine resulted in 4/9 (44%) seroconversion against both viruses. After the first two vaccinations, the seroconversion rates for the HTNV and PUUV vaccines were >80%.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures




References
-
- Hooper, J. W. & Hammerbeck, C. A. Hantavirus Vaccines. New Generation Vaccines, 4th edition. Myron Levine, 905–913 (Informa Healthcare, 2010).
-
- Hooper, J. W. & Li, D. Vaccines against hantaviruses. Curr. Top. Microbiol. Immunol.256, 171–191 (2001). - PubMed
LinkOut - more resources
Full Text Sources

