Nonaqueous Synthesis of Low-Vacancy Chromium Hexacyanochromate
- PMID: 39552129
- PMCID: PMC11615938
- DOI: 10.1021/acs.inorgchem.4c03856
Nonaqueous Synthesis of Low-Vacancy Chromium Hexacyanochromate
Abstract
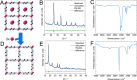
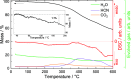
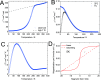
Prussian blue analogues (PBAs) are a highly tunable family of materials with properties suitable for a wide variety of applications. Although their straightforward aqueous synthesis allows for the facile preparation of a diverse set of compositions, the use of water as the solvent has hindered the preparation of specific compositions with highly sought-after properties. A typical example is Cr[Cr(CN)6]: its predicted strong magnetic interactions have motivated many attempts at its synthesis but with limited success. The lack of control over vacancies, crystallinity, and the oxidation state has prevented the experimental validation of its theoretical magnetic properties. Here, we report the nonaqueous synthesis of vacancy-suppressed, nanocrystalline chromium hexacyanochromate. The control over vacancies and the oxidation state leads to stronger magnetic interactions with a markedly increased absolute Weiss temperature (Θ = -836(6) K) and magnetic ordering temperature of (240 ± 10) K. Our results challenge the notion of the solvent as merely reaction medium and introduce a pathway for exploring moisture- and air-sensitive PBA compositions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Tokoro H.; Hashimoto K.; Ohkoshi S.-i. Photo-induced charge-transfer phase transition of rubidium manganese hexacyanoferrate in ferromagnetic and paramagnetic states. J. Magn. Magn. Mater. 2007, 310, 1422–1428. 10.1016/j.jmmm.2006.10.429. - DOI
-
- Holmes S. M.; Girolami G. S. Sol–Gel Synthesis of KV II [Cr III (CN) 6 ]·2H 2 O: A Crystalline Molecule-Based Magnet with a Magnetic Ordering Temperature above 100 °C. J. Am. Chem. Soc. 1999, 121, 5593–5594. 10.1021/ja990946c. - DOI
-
- Néel M. L. Propriétés magnétiques des ferrites; ferrimagnétisme et antiferromagnétisme. Ann. Phys. (Paris). 1948, 12, 137–198. 10.1051/anphys/194812030137. - DOI
LinkOut - more resources
Full Text Sources

