Systemic administration of a viral nanoparticle neoadjuvant prevents lung metastasis development through emergency myelopoiesis
- PMID: 39552216
- PMCID: PMC11581170
- DOI: 10.1080/2162402X.2024.2429846
Systemic administration of a viral nanoparticle neoadjuvant prevents lung metastasis development through emergency myelopoiesis
Abstract
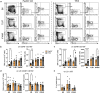
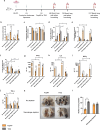
Cancer presents a significant public health concern, particularly in the context of metastatic disease. Surgical removal of primary tumors, while essential, can inadvertently heighten the risk of metastasis. Thus, there is a critical need for innovative neoadjuvant therapies capable of curtailing metastatic progression before or immediately following tumor resection. Addressing this imperative, the papaya mosaic virus nanoparticle (PapMV) has demonstrated potent immunostimulatory capabilities against both viruses and tumors, effectively hindering their proliferation. Our study reveals that PapMV exerts a protective effect against lung metastasis when administered systemically prior to tumor implantation or during the early stages of metastasis in various mouse models of cancer. This anti-tumor effect is initiated by the recruitment of myeloid cells in the lungs. These cells adopt a pro-inflammatory profile, secreting cytokines such as IFN-α, thus fostering a tumor microenvironment inhospitable to tumor progression. Crucially, this protective mechanism hinges on the presence of macrophages before treatment. TLR7 and IFN-I signaling pathways also play pivotal roles in this process. Furthermore, our findings demonstrate that PapMV triggers the activation of the bone marrow emergency response, which accounts for the influx of myeloid cells into the lungs. This study unveils a novel aspect of PapMV's functionality. By bolstering the immune system, PapMV confers robust protection against metastasis at an early stage of disease progression. This discovery holds promise for therapeutic intervention, particularly as a preemptive measure prior to or just after surgical intervention.
Keywords: Cancer immunotherapy; emergency myelopoiesis; metastasis prevention; neoadjuvant therapy; virus-based nanoparticle.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
