Treatment of Critical Bleeds in Patients With Immune Thrombocytopenia: A Systematic Review
- PMID: 39552264
- PMCID: PMC11798764
- DOI: 10.1111/ejh.14351
Treatment of Critical Bleeds in Patients With Immune Thrombocytopenia: A Systematic Review
Abstract
Objectives: Evidence-based protocols for managing bleeding emergencies in patients with immune thrombocytopenia (ITP) are lacking. We conducted a systematic review of treatments for critical bleeding in patients with ITP.
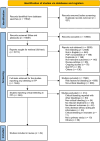
Methods: We included all study designs and extracted data in aggregate or individually for patients who received one or more interventions and for whom any of the following outcomes were reported: platelet count response, bleeding, disability, or death.
Results: We identified 49 eligible studies reporting 112 critical bleed patients with ITP, including 66 children (median age, 10 years), 36 adults (median age, 41.5 years), and 10 patients with unreported age. Patients received corticosteroids (n = 67), IVIG (n = 49), platelet transfusions (n = 41), TPO-RAs (n = 17), and splenectomy (n = 28) either alone or in combination. Studies reported 29 different treatment combinations, the 5 most common were corticosteroids, platelet transfusion and splenectomy (n = 13), corticosteroids and IVIG (n = 13), or splenectomy alone (n = 13); IVIG alone (n = 11); and corticosteroids, IVIG and TPO-RA (n = 8). Mortality among patients with critical bleeds in ITP was 30.6% for adults and 19.7% for children.
Conclusions: The effects of individual treatments on patient outcomes were uncertain due to very low-quality evidence. There is a need for a standardized approach to the treatment of ITP critical bleeds.
Systematic review registration: CRD42020161206.
Keywords: critical bleeding; emergency management; immune thrombocytopenia; major bleeding; thrombocytopenia.
© 2024 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Pai reports personal fees from Pfizer, personal fees and non‐financial support from Pfizer, outside the submitted work; Dr. Beck reports grants from Canadian Institutes of Health Research, during the conduct of the study; Dr. DiRaimo has not personally received any payment, PDSA received grants and consultancy fees from Novartis, Sobi, Sanofi, Amgen and Argenx but not for anything related to this work/manuscript; Dr. Cuker reports personal fees from Synergy, personal fees from Sanofi, personal fees from Pfizer, personal fees from MingSight, personal fees from UpToDate, outside the submitted work; Dr. Grace reports other from Agios, other from Novartis, other from Sobi, other from Sanofi, outside the submitted work; Dr. Arnold reports grants and personal fees from Rigel, personal fees from Amgen, personal fees from Medison, personal fees from Daiichi Sankyo, personal fees from Sobi, personal fees from Principia, personal fees from Chugai, personal fees from Alpine, personal fees from ArgenX, personal fees from Sanofi, personal fees from UpToDate, outside the submitted work. Other authors declare they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical