Incidence of post-acute COVID-19 symptoms across healthcare settings in seven countries: an international retrospective cohort study using routinely-collected data
- PMID: 39552716
- PMCID: PMC11564986
- DOI: 10.1016/j.eclinm.2024.102903
Incidence of post-acute COVID-19 symptoms across healthcare settings in seven countries: an international retrospective cohort study using routinely-collected data
Abstract
Background: The World Health Organisation (WHO) has identified a range of symptomatic manifestations to aid in the clinical diagnosis of post-COVID conditions, herein referred to as post-acute COVID-19 symptoms. We conducted an international network cohort study to estimate the burden of these symptoms in North American, European, and Asian populations.
Methods: A federated analysis was conducted including 10 databases from the United Kingdom, Netherlands, Norway, Estonia, Spain, France, South Korea, and the United States, between September 1st 2020 and latest data availability (which varied from December 31st 2021 to February 28th 2023), covering primary and secondary care, nationwide registries, and claims data, all mapped to the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM). We defined two cohorts for the main analyses: a SARS-CoV-2 infection cohort [positive polymerase chain reaction (PCR) or rapid lateral flow test (LFT) result or clinical COVID-19 diagnosis] and a general population cohort. Individuals with less than 365 days of prior history or 120 days of follow-up were excluded. We estimated incidence rates (IRs) of the 25 WHO-proposed post-acute COVID-19 symptoms, considering symptoms that occurred ≥90 and ≤365 days after index date, excluding individuals with the respective symptoms 180 days prior to the index event. Stratified analyses were conducted by age and sex. Incidence rate ratios (IRRs) were calculated comparing rates in the infected cohort versus the general population. Results from the different databases were combined using random-effects meta-analyses.
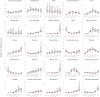
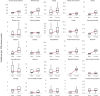
Findings: 3,019,408 individuals were included in the infection cohort. 1,585,160 of them were female and 1,434,248 of them male. 929,351,505 individuals were included in the general population group. 461,195,036 of them were female and 466,022,004 of them male. The 1-year IR of any post-acute COVID-19 symptom in the COVID-19 infection cohort varied significantly across databases, from 4.4 (95% CI 3.8-5.1) per 100 person-years to 103.9 (95% CI 103.2-104.7). The five most common symptoms were joint pain (from 1.6 (95% CI 1.3-1.9) to 14.3 (95% CI 14.1-14.6)), abdominal pain (from 0.3 (95% CI 0.1-0.5) to 9.9 (95% CI 9.7-10.1)), gastrointestinal issues (from 0.6 (95% CI 0.4-0.9) to 13.3 (95% CI 13.1-13.6)), cough (from 0.3 (95% CI 0.2-0.5) to 9.1 (95% CI 8.9-9.3)), and anxiety (from 0.8 (95% CI 0.6-1.2) to 11.4 (95% CI 11.2-11.6)); whereas muscle spasms (from 0.01 (95% CI 0.008-0.2) to 1.7 (95% CI 1.6-1.8)), pins and needles (from 0.05 (95% CI 0.03-0.0.9) to 1.5 (95% CI 1.4-1.6)), memory issues (from 0.03 (95% CI 0.02-0.06) to 0.8 (95% CI 0.7-0.8)), cognitive dysfunction (from 0.007 (95% CI 0.004-0.01) to 0.6 (95% CI 0.4-0.8)), and altered smell and/or taste (from 0.04 (95% CI 0.03-0.04) to 0.7 (95% CI 0.6-0.8)) were least common. Incidence rates of any post-acute COVID-19 symptoms generally increased with age, with certain symptoms peaking in middle-aged adults (anxiety, depressive disorders, headache, altered smell and taste) and others in pre-school children (gastrointestinal issues and cough). Females had higher incidence rates for most symptoms. Based on the random-effects model, the infected cohort had a higher incidence of any post-acute COVID-19 symptom than the general population, with a meta-analytic incidence rate ratio (meta-IRR) of 1.4 (1-2). A similar pattern was seen for all individual symptoms. The highest meta-IRRs were depressive disorder, 2.6 (1.7-3.9); anxiety, 2.3 (1.4-3.8); allergy, 2.1 (1.7-2.8) and sleep disorders, 2.1 (1.5-2.6). The meta-IRR for altered smell and/or taste was 1.9 (1.3-2.8).
Interpretation: Post-acute COVID-19 symptoms, as listed by the WHO, were commonly observed following COVID-19 infection. However, even after standardising research methods, there was significant heterogeneity in the incidence rates from different healthcare settings and geographical locations. This is the first international study of the epidemiology of post-acute COVID-19 symptoms using the WHO-listed symptoms. Its findings contibute to understand the epidemiology of this condition from a multinational approach. Limitations of this study include the lack of consensus of the post-acute COVID-19 definition, as well as the difficulty to capture the impact on daily life of the post-acute COVID-19 symptoms in the available datasets.
Funding: This work has been funded by the European Health Data Evidence Network (EHDEN) through an Evidence Generation Fund Grant and by the National Institute for Health and Care Research (NIHR) Oxford Biomedical Research Centre (BRC).
Keywords: Epidemiology; Incidence of post-acute COVID-19 symptoms; International cohort study; Post-acute COVID-19 condition; Real world data.
© 2024 The Authors.
Conflict of interest statement
D.P.A.‘s department has received grant/s from Amgen, Chiesi–Taylor, Lilly, Janssen, Novartis, and UCB Biopharma. His research group has received consultancy fees from Astra Zeneca and UCB Biopharma. Amgen, Astellas, Janssen, Synapse Management Partners and UCB Biopharma have funded or supported training programmes organised by DPA's department. R.P. reports serving on advisory boards for Gilead Sciences, Inc, Pfizer, Inc, Roche Therapeutics, MSD, GSK, ViiV Healthcare, Eli Lilly and Company, PharmaMar, and Atea Pharmaceuticals, Inc; and receiving research grants paid to his institution from MSD, ViiV Healthcare, Gilead Sciences, and PharmaMar. L.M. reports receiving grants from Grifols; receiving honoraria as a speaker from AstraZeneca, Gilead Sciences, GSK, and Pfizer; and participation in advisory boards for Gilead Sciences and Merck. M.M. works for a research group that in the past 3 years received unconditional research grants from Chiesi, UCB, Amgen, Johnson & Johnson, Innovative Medicines Initiative and the European Medicines Agency. D.Ded., Z.C. and J.O. are employees of the Medicines and Healthcare Products Regulatory Agency, which provides the CPRD research service. K.K. is a consortial author in the US National Institutes of Health National COVID Cohort Collaborative (funding expired in 2022 with no renewal or active impact on any current work). G.M. reports receiving consulting fees from Pfizer. A.N. reports grants or contracts from Alfred P. Sloan Foundation, National Institutes of Health and the U.S. Food and Drug Administration. L.P. was supported by a Sara Borrell fellowship awarded by the Spanish Institute of Health Carlos III (CD23/00223). K.L.G is funded through an MRC scholarship with Bayer AG as an industrial partner. All other co-authors declare no competing interests.
Figures


References
-
- Nalbandian A., Desai A.D., Wan E.Y. Post-COVID-19 condition. Annu Rev Med. 2023;74:55–64. - PubMed
-
- Crook H., Raza S., Nowell J., Young M., Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. - PubMed
-
- Hodgson C.L., Broadley T. Long COVID—unravelling a complex condition. Lancet Respir Med. 2023;11:667–668. - PubMed
-
- COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE); London: 2020. - PubMed
-
- Carcaillon-Bentata L., Makovski T.T., Alleaume C., et al. Post-Covid-19 condition: a comprehensive analysis of the World Health Organisation definition. J Infect. 2023;87:e83–e87. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

