Real world effectiveness of early ensitrelvir treatment in patients with SARS-CoV-2, a retrospective case series
- PMID: 39552926
- PMCID: PMC11567130
- DOI: 10.1016/j.nmni.2024.101522
Real world effectiveness of early ensitrelvir treatment in patients with SARS-CoV-2, a retrospective case series
Abstract
Background: Ensitrelvir, a 3C-like protease inhibitor, received emergency approval in Japan in November 2022 for treating non-hospitalized patients with mild-to-moderate COVID-19. However, confirmation of its real-world clinical effectiveness is limited.
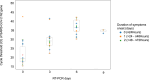
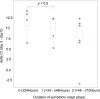
Methods: This retrospective study evaluated 18 vaccinated outpatients (15 men; median age, 39.5 years; range, 26-56), treated with a 5-day oral ensitrelvir regimen (375 mg loading dose, followed by 125 mg daily) between December 1, 2022, and January 31, 2023. Nasal swabs were collected on days 0, 3, 6, and 9 for RT-qPCR to assess viral load. Variants were identified by Sanger sequencing, and outcomes were compared to historical controls. Patients were followed for 60 days to monitor for post-acute sequelae of COVID-19 (PASC).
Results: Symptoms such as mild fever and sore throat improved rapidly after one day of ensitrelvir treatment, with 66 % of patients recovering within six days. All individuals were infected with the BA.5 Omicron variant. Viral loads, as measured by Ct values, increased significantly from 21.82 at symptom onset to 37.65 b y day 6, with SARS-CoV-2 RNA undetectable in most patients by day 9. Those treated within 48 h of symptom onset showed the viral load reduction. Compared to historical controls, where symptom resolution took 8.5 days, ensitrelvir shortened recovery time to as little as 1.4 days for over 66 % of patients.
Conclusion: Ensitrelvir treatment resulted in rapid symptom relief and significant viral load reduction, with no adverse events, viral rebound, or PASC symptoms, demonstrating its potential efficacy and safety. Larger studies are needed for further confirmation.
© 2024 The Authors.
Conflict of interest statement
No author declares any potential conflict of interest or competing financial or non-financial interest in relation to the manuscript.
Figures

References
-
- Wannigama D.L., Hurst C., Phattharapornjaroen P., Hongsing P., Sirichumroonwit N., Chanpiwat K., et al. Early treatment with fluvoxamine, bromhexine, cyproheptadine, and niclosamide to prevent clinical deterioration in patients with symptomatic COVID-19: a randomized clinical trial. eClinicalMedicine. 2024;70 - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
