Sodium Polystyrene Sulfonate-Induced Massive Bowel Necrosis With Distant Extraintestinal Crystal Deposition: A Case Report and Review of the Literature
- PMID: 39553032
- PMCID: PMC11563774
- DOI: 10.7759/cureus.71523
Sodium Polystyrene Sulfonate-Induced Massive Bowel Necrosis With Distant Extraintestinal Crystal Deposition: A Case Report and Review of the Literature
Abstract
Sodium polystyrene sulfonate (SPS), a cation-exchange resin, has been a mainstay in long-term hyperkalemia management but is associated with significant gastrointestinal complications, particularly when used with sorbitol. The deposition of SPS crystals within the intestinal mucosa has been suggested to precipitate ischemia, necrosis, and ulcerations, ultimately leading to bowel perforation. This case report details a striking instance of massive bowel perforation subsequent to SPS administration, with accompanying findings of disseminated crystals in distant organs and tissues upon autopsy. Additionally, we provide a comprehensive review of the existing literature on this rare yet significant drug-induced side effect.
Keywords: autopsy findings; bowel perforation; cation-exchange resin; drug-induced bowel injury; extraintestinal crystal deposition; sodium polystyrene sulfonate.
Copyright © 2024, Brezic et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures


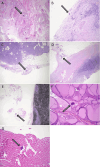
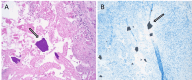
References
-
- Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM. Am J Med. 2013;126:264. - PubMed
-
- Upper gastrointestinal tract injury in patients receiving Kayexalate (sodium polystyrene sulfonate) in sorbitol: clinical, endoscopic, and histopathologic findings. Abraham SC, Bhagavan BS, Lee LA, Rashid A, Wu TT. Am J Surg Pathol. 2001;25:637–644. - PubMed
-
- Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Watson MA, Baker TP, Nguyen A, et al. Am J Kidney Dis. 2012;60:409–416. - PubMed
-
- Intestinal necrosis related to administration of cation exchange resin without sorbitol: a retrospective analysis of 61 patients with end-stage renal diseases. Murakami K, Nakamura Y, Miyasaka Y, et al. Pathol Int. 2020;70:270–279. - PubMed
-
- Necrotizing enterocolitis in a 850 gram infant receiving sorbitol-free sodium polystyrene sulfonate (Kayexalate): clinical and histopathologic findings. Rugolotto S, Gruber M, Solano PD, Chini L, Gobbo S, Pecori S. J Perinatol. 2007;27:247–249. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
