Importance of Ultraviolet-C (UV-C) Emitter Configuration for the Attenuation of Staphylococcus aureus and Candida auris Pathogens
- PMID: 39553089
- PMCID: PMC11566098
- DOI: 10.7759/cureus.71612
Importance of Ultraviolet-C (UV-C) Emitter Configuration for the Attenuation of Staphylococcus aureus and Candida auris Pathogens
Abstract
Background: The relative importance of different ultraviolet-C (UV-C) emitter configurations on the attenuation of vegetative bacterial and fungal pathogens has not been assessed. We hypothesized that emitter configuration would impact the efficacy of UV-C attenuation of Staphylococcus aureus ( S. aureus) and Candida auris (C. auris) pathogens.
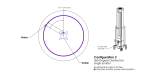
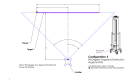
Methods: American Type Culture Collection (ATCC) S. aureus (ATCC 6538) and C. auris (ATCC MYA-5001) carriers (ReadyNowTM Test Carriers, Stratix Labs Corporation, Saint Paul, MN) were mounted on an aluminum stand along with three calibrated radiometers (International Light Technologies model ILT1270, Peabody, MA). Five UV-C emitter configurations were assessed, including three emitters with a triangular configuration about the stand and each rotating 360° (1), one emitter facing the stand and rotating 360° (2), three emitters facing the stand in a linear configuration and each rotating 5° (3), one emitter facing the stand and rotating 5° (4), and one emitter facing the stand and rotating 90° (5). Three serial experiments were conducted. The first experiment involved the establishment of the minimally effective irradiation dose (mean and standard deviation mJ/cm2) required to achieve no growth (6-log reduction (LR)) with direct exposure to pathogen carriers positioned at the center of the lamp. We then assessed the relative efficacy of delivery of the minimally effective dose via the five emitter configurations in attenuating polycarbonate and textured pathogen carriers. Polycarbonate carriers were positioned at 25.5 and 69.5 inches from the floor and oriented vertically to the emitters. Textured plastic pathogen carriers were positioned at 47.5 or 58.5 inches from the floor and with a 45° or horizontal orientation to the emitters. Standard carriers (1"x0.9") were used for both pathogens and large carriers (1"x3") for C. auris,the latter to address the potential for cell clustering.
Results: With standard carriers, the minimally effective dose was 27.01± 0.15 mJ/cm2 for S. aureus but was not achieved for C. auris. The minimally effective dose for large C. auris carriers was 596.62 ± 27.98 mJ/cm2. With standard carriers, all configurations achieved a >6 log reduction for S. aureus, and none achieved a >6 log reduction for C. auris. All configurations achieved a > 6 log reduction when 596.62 ± 27.98 mJ/cm2 was delivered to large C. auris carriers. Changing to textured plastic carriers (standard for S. aureus and large for C. auris) and varying height (47.5-69.5 inches) from the floor and orientation to the emitters (45° and horizontal), the mean ± standard deviation for S. aureus and C. auris log reductions with delivery of the minimally effective dose was 4.44 ± 2.02, 2.58 ± 2.37, 3.55 ± 2.67, 2.33 ± 2.47, and 3.00 ± 2.64 for configurations one through five, respectively. Configuration one achieved a significantly greater LR than configurations two (adjusted P = 0.0018) and four (adjusted P = 0.023). There were 22% (6/27) of sites ≥ 100 colony-forming units (CFU) following cleaning but before UV-C vs. 0% (0/27) of sites ≥ 100 CFU after surface disinfection cleaning and nine minutes of configuration three UV-C treatment (P = 0.023).
Conclusions: The choice of UV-C emitter configuration can impact S. aureus and C. auris attenuation when there is indirect exposure to the pathogen. Emitter configuration should be considered as an important parameter for future UV-C technological assessments.
Keywords: candida auris; emitter configuration; pathogen attenuation; staphylococcus aureus; ultraviolet-c (uv-c); uv-c emitters.
Copyright © 2024, Loftus et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: Surfacide Manufacturing provided support. Financial relationships: Dr. Loftus declare(s) a grant from Surfacide. Dr. Loftus declare(s) a grant from Draeger. Dr. Loftus declare(s) a grant and support for meeting from Kenall. Dr. Loftus declare(s) a grant and support for meeting from BBraun. Dr. Dexter declare(s) Dr. Dexter is Director of the Division of Management Consulting of the University of Iowa Department of Anesthesia, which provides consultations to corporations, hospitals, and individuals, including RDB Bioinformatics. He receives no funds personally other than his salary and allowable expense reimbursements from the University of Iowa. His family and he have no financial holdings in any company related to his work. A list of all the Division’s consults is available in his posted curriculum vitae at https://FranklinDexter.net/Contact_Info.htm. from University of Iowa. Dr. Seering declare(s) personal fees from GE. Dr. Seering received payment from GE for her involvement in an unrelated study (ETControl). Dr. Brent Hadder declare(s) Expert testimony from Law firm. Has received payment for expert testimony unrelated to this submission. Randy W. Loftus, Michelle C. Parra declare(s) a grant, a patent and stock/stock options from RDB Bioinformatics. Dr. Loftus received research funding from Sage Medical Inc., BBraun, Draeger, Surfacide and Kenall, has one or more patents pending, and is a partner of RDB Bioinformatics, LLC, at 1055 N 115th St #301 (Omaha, NE, USA), the company that owns OR PathTrac. He receives no funds personally from his involvement in RDB. He has spoken at educational meetings sponsored by Kenall and BBraun. Dr. Parra is affiliated with RDB Bioinformatics as Dr. Loftus's spouse. Dr. Parra has no other conflicts. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures




References
-
- Use of ultraviolet-C in environmental sterilization in hospitals: a systematic review on efficacy and safety. Ramos CC, Roque JL, Sarmiento DB, et al. https://pubmed.ncbi.nlm.nih.gov/33192232/ Int J Health Sci (Qassim) 2020;14:52–65. - PMC - PubMed
-
- Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Datta R, Platt R, Yokoe DS, Huang SS. Arch Intern Med. 2011;171:491–494. - PubMed
-
- Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Anderson DJ, Chen LF, Weber DJ, et al. Lancet. 2017;389:805–814. - PMC - PubMed
-
- Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR Disinfection) Anderson DJ, Moehring RW, Weber DJ, et al. Lancet Infect Dis. 2018;18:845–853. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
