Cabozantinib inhibits tumor growth in mice with ovarian cancer
- PMID: 39553221
- PMCID: PMC11560812
- DOI: 10.62347/ZSWV1767
Cabozantinib inhibits tumor growth in mice with ovarian cancer
Abstract
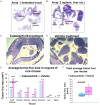
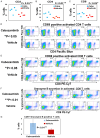
Ovarian cancer is usually detected in the advanced stages. Existing treatments for high grade serous ovarian cancer (HGSOC) are not adequate and approximately fifty percent of patients succumb to this disease and die within five years after diagnosis. We conducted pre-clinical studies in a mouse model of ovarian cancer to evaluate disease outcome in response to treatment with the multi-kinase inhibitor cabozantinib. Cabozantinib is a receptor tyrosine kinase inhibitor with multiple targets including vascular endothelial growth factor receptor-2 (VEGFR-2), associated with immune suppression in ovarian cancer. Mice (C57BL/6) were injected with ID8-RFP ovarian tumor cells and treated with cabozantinib. Studies investigated ascites development, tumor burden and regulation of anti-tumor immunity with treatment. Mice treated with cabozantinib had significantly decreased solid tumor burden and decreased malignant ascites as compared to untreated controls. Improved outcome in cabozantinib treated mice was associated with a significantly higher percentage of CD69 early activated T cells, a higher percentage of granzyme B secreting CD8 T cells, the enhanced release of cytokines and chemokines known to recruit CD8 T cells and amplify T cell function, as well as reduced VEGFR-2. Findings suggest that cabozantinib is an important clinical agent capable of improving ovarian cancer in mice potentially in part by priming the autologous immune system to promote anti-tumor immunity.
Keywords: Ovarian cancer; anti-tumor immunity; cabozantinib treatment; disease improvement; mouse model.
AJCR Copyright © 2024.
Conflict of interest statement
None.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280–304. - PubMed
-
- Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol. 2017;34:103. - PubMed
-
- Zheng L, Cui C, Shi O, Lu X, Li YK, Wang W, Li Y, Wang Q. Incidence and mortality of ovarian cancer at the global, regional, and national levels, 1990-2017. Gynecol Oncol. 2020;159:239–247. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
