Single immunization with an influenza hemagglutinin nanoparticle-based vaccine elicits durable protective immunity
- PMID: 39553436
- PMCID: PMC11561850
- DOI: 10.1002/btm2.10689
Single immunization with an influenza hemagglutinin nanoparticle-based vaccine elicits durable protective immunity
Abstract
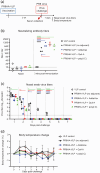
Vaccination is the most effective strategy to combat influenza. Ideally, potent and persistent vaccine effects would be induced with a single vaccine dose. Here, we designed a virus-like particle (VLP)-based vaccine presenting multiple copies of the influenza hemagglutinin (HA) from A/Puerto Rico/8/1934 (PR8HA-VLP) and examined its immunogenicity and protective efficacy in ferrets. Serum-neutralizing antibodies were effectively induced against the homologous virus at 3-week post-vaccination with a single dose of PR8HA-VLP with or without adjuvants. When the single-immunized ferrets were challenged with the homologous virus, virus replication in the nasal mucosa was significantly reduced. Long-term monitoring of serum titers revealed that after adjuvanted vaccination with PR8HA-VLP, neutralizing antibodies were retained at similar levels 20- to 183-week post-vaccination, although a 4- to 8-fold titer decline was observed from 3- to 20-week post-vaccination. Boost immunization at 183 weeks after the first immunization elicited higher neutralizing antibody titers than those at 3 weeks after the initial immunization in most of the animals. These results confirm that nanoparticle-based vaccines are a promising approach to effectively elicit durable multiyear neutralizing antibody responses against influenza viruses.
Keywords: VLP‐based vaccine; adjuvant; duration of immunity; ferret; influenza A virus; neutralizing antibody titers; virus‐like particles (VLPs).
© 2024 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
YK has received unrelated funding support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. LTD, Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio. YK is a co‐founder of FluGen. The remaining authors have no conflicts of interest to declare.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

