The effect of fibroblast growth factor 2 on neovascular vessels depends on the stage of angiogenesis
- PMID: 39553576
- PMCID: PMC11566843
- DOI: 10.1016/j.heliyon.2024.e39843
The effect of fibroblast growth factor 2 on neovascular vessels depends on the stage of angiogenesis
Abstract
Objective: The exact relationship between fibroblast growth factor 2 (FGF2) and choroidal neovascularization (CNV) remains unclear. In this study, using optical coherence tomography angiography (OCTA) and FGF2-tg mice which are transgenic mice with a rhodopsin promoter/FGF2 gene fusion, we aimed to investigate the dynamics of FGF2's role in angiogenesis over time.
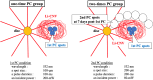
Methods: We developed laser-induced CNV models of FGF2-tg and wild-type (WT) mice and then separated them into two groups using different laser photocoagulation (PC) conditions. The first group received 3 intense PC shots (1st PC) altogether (one-time PC group), while the other group received 3 intense PC shots (1st PC) followed by 6 additional weak PC shots (2 nd PC) on the 7th day after 1st PC (two-times PC group).
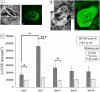
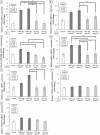
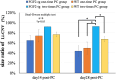
Results: Using OCTA to observe vessel changes within the same individual over time, there was no difference in the timing of vessel transition from the CNV development phase to the CNV regression phase between FGF2-tg and WT mice in the one-time PC group. In contrast, the neovascular vessels in the two-times PC group of FGF2-tg mice were maintained at least 28 days post-2nd PC without regression. In addition, mature vessels surrounded by PDGFRβ positive pericytes and α-SMA positive smooth muscle cells were observed. Real-time qPCR showed a substantial increase in apelin mRNA expression in the one-time PC group of FGF2-tg, rather than VEGF-A (p < 0.05, n = 5 or 6). Moreover, the expression levels of PDGFRβ, apelin, and Ang1 were significantly higher in FGF2-tg mice of two-times PC group than in WT mice (p < 0.05, n = 5 or 6).
Conclusions: FGF2 not only promotes neovascularization via the apelin/APJ system, which is independent of VEGF signaling pathway, but also helps maintain and stabilize pre-existing neovascular vessels by stimulating PDGFRβ and Ang1. The effect of FGF2 on the neovascular vessels depends on the stage of angiogenesis.
Keywords: Ang1; Apelin; FGF2; Intravitreal anti-VEGF treatment; OCTA; PDGFRβ; Vessels maturation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Seghezzi G., Patel S., Ren C.J., et al. Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J. Cell Biol. 1998;141:1659–1673. doi: 10.1083/jcb.141.7.1659. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

