Chiral amido-oxazoline functionalized MCM-41: A sustainable heterogeneous catalyst for enantioselective Kharasch-Sosnovsky and Henry reactions
- PMID: 39553607
- PMCID: PMC11565425
- DOI: 10.1016/j.heliyon.2024.e39911
Chiral amido-oxazoline functionalized MCM-41: A sustainable heterogeneous catalyst for enantioselective Kharasch-Sosnovsky and Henry reactions
Abstract
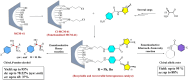
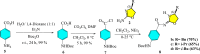
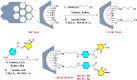
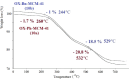
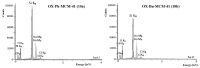
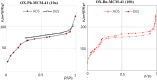
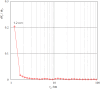
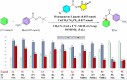
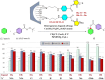
In this study, a series of chiral amido-oxazoline ligands was synthesized with a primary focus on immobilizing the most effective ligands on MCM-41 mesoporous material. Following several attempts, the para-nitro group of the chiral amido-oxazoline ligands was successfully reduced to amino group, enabling their immobilization on MCM-41. The resulting chiral heterogeneous amido-oxazoline ligands were characterized using various techniques, including FT-IR, XRD, TGA, SEM, TEM, EDX, and BET-BJH, confirming the successful immobilization of the amido-oxazoline ligands. A comparison of the efficiency of the homogeneous and heterogeneous amido-oxazoline-based ligands in the Kharasch-Sosnovsky and Henry reactions revealed better performance of the heterogeneous ligand. The immobilized amido-oxazoline-copper complexes exhibited remarkable catalytic activity, achieving excellent yields and enantioselectivities (up to 88 % ee) in the Kharasch-Sosnovsky reaction, and delivering excellent yields with moderate enantioselectivities in the Henry reaction. Notably, the Henry reaction proceeded with moderate diastereoselectivity, favoring the syn diastereomer, under solvent-free conditions, highlighting the sustainability of the process. The heterogeneous nature of the catalysts facilitated effortless recovery and efficient reusability.
Keywords: Amido-oxazoline ligands; Enantioselective Henry reaction; Enantioselective Kharasch–Sosnovsky reaction; Mesoporous MCM-41; Sustainable heterogeneous catalyst.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


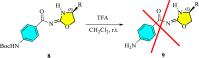
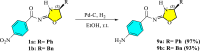







References
-
- Braunstein P., Naud F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline‐based systems. Angew. Chem. Int. Ed. 2001;40:680–699. - PubMed
-
- Gómez M., Muller G., Rocamora M. Coordination chemistry of oxazoline ligands. Coord. Chem. Rev. 1999;193:769–835.
-
- Cryder J.L., Killgore A.J., Moore C., Golen J.A., Rheingold A.L., Daley C.J. Novel metal complexes containing a chiral trinitrogen isoindoline-based pincer ligand: in situ synthesis and structural characterization. Dalton Trans. 2010;39:10671–10677. - PubMed
-
- Fraile J.M., García J.I., Herrerías C.I., Mayoral J.A., Reiser O., Socuéllamos A., Werner H. The role of binding constants in the efficiency of chiral catalysts immobilized by electrostatic interactions: the case of azabis (oxazoline)–copper complexes. Chem. Eur. J. 2004;10:2997–3005. - PubMed
-
- Gissibl A., Finn M., Reiser O. Cu(II)-Aza (bisoxazoline)-catalyzed asymmetric benzoylations. Org. Lett. 2005;7:2325–2328. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

