Applications of microfluidics in mRNA vaccine development: A review
- PMID: 39553921
- PMCID: PMC11567697
- DOI: 10.1063/5.0228447
Applications of microfluidics in mRNA vaccine development: A review
Abstract
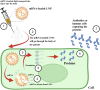
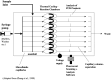
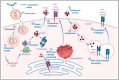
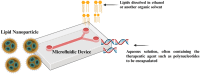
The transformative potential of microfluidics in the development of mRNA vaccines is explored in this review, highlighting its pivotal role in enhancing easy-to-use functionality, efficacy, and production efficiency. Moreover, we examine the innovative applications of microfluidics in biomedical research, including its contribution to the rapid and cost-effective synthesis of lipid nanoparticles for mRNA delivery and delve into the advantages of mRNA vaccines, such as targeted delivery and controlled expression. Furthermore, it outlines the future prospects of microfluidic devices, their cutting-edge examples in both research and industry, and the potential to revolutionize vaccine formulation and production. The integration of microfluidics with mRNA vaccine development represents a significant advancement in public health and disease prevention strategies.
© 2024 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures



References
-
- Sharp P. A., “Split genes and RNA splicing (nobel lecture),” Angew. Chem., Int. Ed. Engl. 33(12), 1229–1240 (1994). 10.1002/anie.199412291 - DOI
Publication types
LinkOut - more resources
Full Text Sources
