This is a preprint.
DNMT3AR882H Is Not Required for Disease Maintenance in Primary Human AML, but Is Associated With Increased Leukemia Stem Cell Frequency
- PMID: 39553934
- PMCID: PMC11565803
- DOI: 10.1101/2024.10.26.620318
DNMT3AR882H Is Not Required for Disease Maintenance in Primary Human AML, but Is Associated With Increased Leukemia Stem Cell Frequency
Update in
-
DNMT3A R882H Is Not Required for Disease Maintenance in Primary Human AML, but Is Associated With Increased Leukemia Stem Cell Frequency.Cancer Discov. 2025 Nov 20:10.1158/2159-8290.CD-24-1604. doi: 10.1158/2159-8290.CD-24-1604. Online ahead of print. Cancer Discov. 2025. PMID: 41263425 Free PMC article.
Abstract
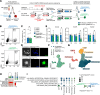
Genetic mutations are being thoroughly mapped in human cancers, yet a fundamental question in cancer biology is whether such mutations are functionally required for cancer initiation, maintenance of established cancer, or both. Here, we study this question in the context of human acute myeloid leukemia (AML), where DNMT3A R882 missense mutations often arise early, in pre-leukemic clonal hematopoiesis, and corrupt the DNA methylation landscape to initiate leukemia. We developed CRISPR-based methods to directly correct DNMT3A R882 mutations in leukemic cells obtained from patients. Surprisingly, DNMT3A R882 mutations were largely dispensable for disease maintenance. Replacing DNMT3A R882 mutants with wild-type DNMT3A did not impair the ability of AML cells to engraft in vivo, and minimally altered DNA methylation. Taken together, DNMT3A R882 mutations are initially necessary for AML initiation, but are largely dispensable for disease maintenance. The notion that initiating oncogenes differ from those that maintain cancer has important implications for cancer evolution and therapy.
Conflict of interest statement
Declaration of interests: R.M. is on the Advisory Boards of Kodikaz Therapeutic Solutions, Orbital Therapeutics, Pheast Therapeutics, 858 Therapeutics, Prelude Therapeutics, Mubadala Capital, and Aculeus Therapeutics. R.M. is a co-founder and equity holder of Pheast Therapeutics, MyeloGene, and Orbital Therapeutics.
Figures



References
-
- Dunbar A.J., Bowman R.L., Park Y.C., O’Connor K., Izzo F., Myers R.M., Karzai A., Zaroogian Z., Kim W.J., Fernandez-Maestre I., et al. (2024). Jak2V617F Reversible Activation Shows Its Essential Requirement in Myeloproliferative Neoplasms. Cancer Discov 14, 737–751. 10.1158/2159-8290.CD-22-0952. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
