This is a preprint.
Type-II kinase inhibitors that target Parkinson's Disease-associated LRRK2
- PMID: 39554022
- PMCID: PMC11565912
- DOI: 10.1101/2024.09.17.613365
Type-II kinase inhibitors that target Parkinson's Disease-associated LRRK2
Update in
-
Type II kinase inhibitors that target Parkinson's disease-associated LRRK2.Sci Adv. 2025 Jun 6;11(23):eadt2050. doi: 10.1126/sciadv.adt2050. Epub 2025 Jun 4. Sci Adv. 2025. PMID: 40465731 Free PMC article.
Abstract
Aberrant increases in kinase activity of leucine-rich repeat kinase 2 (LRRK2) are associated with Parkinson's disease (PD). Numerous LRRK2-selective type-I kinase inhibitors have been developed and some have entered clinical trials. In this study, we present the first LRRK2-selective type-II kinase inhibitors. Targeting the inactive conformation of LRRK2 is functionally distinct from targeting the active-like conformation using type-I inhibitors. We designed these inhibitors using a combinatorial chemistry approach fusing selective LRRK2 type-I and promiscuous type-II inhibitors by iterative cycles of synthesis supported by structural biology and activity testing. Our current lead structures are selective and potent LRRK2 inhibitors. Through cellular assays, cryo-electron microscopy structural analysis, and in vitro motility assays, we show that our inhibitors stabilize the open, inactive kinase conformation. These new conformation-specific compounds will be invaluable as tools to study LRRK2's function and regulation, and expand the potential therapeutic options for PD.
Conflict of interest statement
Competing interest statement Authors declare that they have no competing interests.
Figures
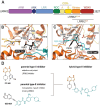

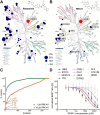
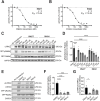


References
-
- Willis A. W., Roberts E., Beck J. C., Fiske B., Ross W., Savica R., Van Den Eeden S. K., Tanner C. M., Marras C., on behalf of the Parkinson’s Foundation P4 Group, Alcalay R., Schwarzschild M., Racette B., Chen H., Church T., Wilson B., Doria J. M., Incidence of Parkinson disease in North America. npj Parkinsons Dis. 8, 170 (2022). - PMC - PubMed
-
- Paisán-Ruíz C., Jain S., Evans E. W., Gilks W. P., Simón J., Van Der Brug M., De Munain A. L., Aparicio S., Gil A. M., Khan N., Johnson J., Martinez J. R., Nicholl D., Carrera I. M., Peňa A. S., De Silva R., Lees A., Martí-Massó J. F., Pérez-Tur J., Wood N. W., Singleton A. B., Cloning of the Gene Containing Mutations that Cause PARK8-Linked Parkinson’s Disease. Neuron 44, 595–600 (2004). - PubMed
-
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B., Stoessl A. J., Pfeiffer R. F., Patenge N., Carbajal I. C., Vieregge P., Asmus F., Müller-Myhsok B., Dickson D. W., Meitinger T., Strom T. M., Wszolek Z. K., Gasser T., Mutations in LRRK2 Cause Autosomal-Dominant Parkinsonism with Pleomorphic Pathology. Neuron 44, 601–607 (2004). - PubMed
-
- Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., Toda T., Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet 41, 1303–1307 (2009). - PubMed
-
- Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M. A., Illig T., Gieger C., Houlden H., Steffens M., Okun M. S., Racette B. A., Cookson M. R., Foote K. D., Fernandez H. H., Traynor B. J., Schreiber S., Arepalli S., Zonozi R., Gwinn K., Van Der Brug M., Lopez G., Chanock S. J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N. W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J. A., Singleton A. B., Gasser T., Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 41, 1308–1312 (2009). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
