This is a preprint.
Dynamic imbalances in cell-type specific striatal ensemble activity during visually guided locomotion
- PMID: 39554032
- PMCID: PMC11565797
- DOI: 10.1101/2024.10.29.620847
Dynamic imbalances in cell-type specific striatal ensemble activity during visually guided locomotion
Abstract
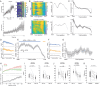
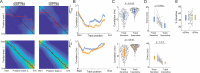
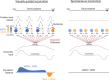
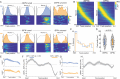
Locomotion is continuously regulated by an animal's position within an environment relative to goals. Direct and indirect pathway striatal output neurons (dSPNs and iSPNs) influence locomotion, but how their activity is naturally coordinated by changing environments is unknown. We found, in head-fixed mice, that the relative balance of dSPN and iSPN activity was dynamically modulated with respect to position within a visually-guided locomotor trajectory to retrieve reward. Imbalances were present within ensembles of position-tuned SPNs which were sensitive to the visual environment. Our results suggest a model in which competitive imbalances in striatal output are created by learned associations with sensory input to shape context dependent locomotion.
Figures

References
-
- Mink J. W. THE BASAL GANGLIA: FOCUSED SELECTION AND INHIBITION OF COMPETING MOTOR PROGRAMS. Prog. Neurobiol. 50, 381–425 (1996). - PubMed
-
- Graybiel A. M., Aosaki T., Flaherty A. W. & Kimura M. The basal ganglia and adaptive motor control. Science 265, 1826–1831 (1994). - PubMed
-
- Robbe D. To move or to sense? Incorporating somatosensory representation into striatal functions. Curr. Opin. Neurobiol. 52, 123–130 (2018). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
