This is a preprint.
Single-cell DNA methylome and 3D genome atlas of the human subcutaneous adipose tissue
- PMID: 39554055
- PMCID: PMC11566006
- DOI: 10.1101/2024.11.02.621694
Single-cell DNA methylome and 3D genome atlas of the human subcutaneous adipose tissue
Update in
-
Single-cell DNA methylome and 3D genome atlas of human subcutaneous adipose tissue.Nat Genet. 2025 Sep;57(9):2238-2249. doi: 10.1038/s41588-025-02300-4. Epub 2025 Aug 20. Nat Genet. 2025. PMID: 40835891 Free PMC article.
Abstract
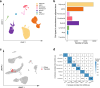
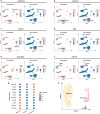
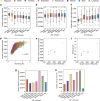
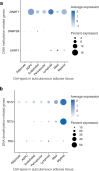
Human subcutaneous adipose tissue (SAT) contains a diverse array of cell-types; however, the epigenomic landscape among the SAT cell-types has remained elusive. Our integrative analysis of single-cell resolution DNA methylation and chromatin conformation profiles (snm3C-seq), coupled with matching RNA expression (snRNA-seq), systematically cataloged the epigenomic, 3D topology, and transcriptomic dynamics across the SAT cell-types. We discovered that the SAT CG methylation (mCG) landscape is characterized by pronounced hyper-methylation in myeloid cells and hypo-methylation in adipocytes and adipose stem and progenitor cells (ASPCs), driving nearly half of the 705,063 detected differentially methylated regions (DMRs). In addition to the enriched cell-type-specific transcription factor binding motifs, we identified TET1 and DNMT3A as plausible candidates for regulating cell-type level mCG profiles. Furthermore, we observed that global mCG profiles closely correspond to SAT lineage, which is also reflected in cell-type-specific chromosome compartmentalization. Adipocytes, in particular, display significantly more short-range chromosomal interactions, facilitating the formation of complex local 3D genomic structures that regulate downstream transcriptomic activity, including those associated with adipogenesis. Finally, we discovered that variants in cell-type level DMRs and A compartments significantly predict and are enriched for variance explained in abdominal obesity. Together, our multimodal study characterizes human SAT epigenomic landscape at the cell-type resolution and links partitioned polygenic risk of abdominal obesity to SAT epigenome.
Figures










References
-
- González-Muniesa P. et al. Obesity. Nat Rev Dis Primers 3, 17034 (2017). - PubMed
-
- Després J.-P. Abdominal obesity: the most prevalent cause of the metabolic syndrome and related cardiometabolic risk. European Heart Journal Supplements 8, B4–B12 (2006).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
