This is a preprint.
The persistent effects of predator odor stressor enhance interoceptive sensitivity to alcohol through GABAA receptor adaptations in the prelimbic cortex in male, but not female rats
- PMID: 39554092
- PMCID: PMC11565848
- DOI: 10.1101/2024.10.30.621141
The persistent effects of predator odor stressor enhance interoceptive sensitivity to alcohol through GABAA receptor adaptations in the prelimbic cortex in male, but not female rats
Abstract
Background: Traumatic stress is associated with high rates of problematic alcohol use, but how the persistent effects of trauma impact sensitivity to alcohol remain unknown. This study examined the persistent effects of traumatic stress exposure on sensitivity to alcohol and underlying neurobiological mechanisms in rats.
Methods: Male (N=98) and female (N=98) Long-Evans rats were exposed to the predator odor TMT, and two weeks later, molecular, neuronal, and behavioral sensitivity to alcohol were assessed. Next, rats were trained to discriminate alcohol from water (male N=70; female N=56), and the impact of TMT on interoceptive sensitivity to alcohol and the alcohol-like effects of systemic GABAA receptor activation were evaluated. Lastly, functional involvement of GABAA and NMDA receptors in the prelimbic cortex (PrL) and the anterior insular cortex (aIC) was investigated.
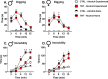
Results: TMT exposure sex-dependently altered PrL Gabra1, and elevated aIC Grin2b and Grin2c in males. TMT increased PrL c-Fos in males, which was attenuated by alcohol administration. Alcohol-induced locomotor and startle response effects were attenuated in the TMT group in both sexes. TMT exposure potentiated interoceptive sensitivity to alcohol in males but not in females, and this effect was driven by GABAA receptors in the PrL. Greater stress reactivity during TMT exposure was associated with higher interoceptive sensitivity to alcohol, and alcohol exposure history was linked to a heightened stress response to TMT in males.
Conclusions: Traumatic stress increased interoceptive sensitivity to alcohol in males, but not females, through PrL GABAA receptor adaptations, potentially enhancing the stimulatory, and by extension the rewarding, effects of alcohol.
Keywords: 2,5-dihydro-2,4,5-trimethylthiazoline; PTSD; alcohol use disorder; anterior insular cortex; drug discrimination; individual differences.
Conflict of interest statement
Disclosures All authors have no conflicts of interest to disclose.
Figures






References
-
- Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. - PubMed
-
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime Co-occurrence of DSM-III-R Alcohol Abuse and Dependence With Other Psychiatric Disorders in the National Comorbidity Survey. Archives of General Psychiatry. 1997;54(4):313–21. - PubMed
-
- Makhijani VH, Franklin JP, Van Voorhies K, Fortino B, Besheer J. The synthetically produced predator odor 2,5-dihydro-2,4,5-trimethylthiazoline increases alcohol self-administration and alters basolateral amygdala response to alcohol in rats. Psychopharmacology (Berl). 2021;238(1):67–82. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
