This is a preprint.
Collagen binding adhesin restricts Staphylococcus aureus skin infection
- PMID: 39554114
- PMCID: PMC11565922
- DOI: 10.1101/2024.11.01.621145
Collagen binding adhesin restricts Staphylococcus aureus skin infection
Abstract
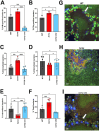
Staphylococcus aureus causes approximately 80% of skin and soft tissue infections (SSTIs). Collagen is the most abundant human extracellular matrix protein with critical roles in wound healing, and S. aureus encodes a collagen binding adhesin (Cna). The role of this protein during skin infections is unknown. Here we report that inability to bind collagen results in worsened pathology of intradermal Δcna S. aureus infection. WT/Cna+ S. aureus showed reduced infection severity, aggregate formation, and significantly improved clearance of bacteria. Cna binds to the collagen-like domain of serum C1q protein to reduce its opsonophagocytic functions. We demonstrate that infection of C1qKO mice with WT bacteria show results similar to the Δcna group. Conversely, inability to bind collagen resulted in an amplified inflammatory response caused in part by macrophage and neutrophil small molecule mediators released at the infection site (MMP-9, MMP-12, LTB4), resulting in increased immune cell infiltration and death.
Keywords: C1q; Collagen; MRSA; inflammation; skin.
Conflict of interest statement
Declaration of interests. The authors declare no competing interests.
Figures





References
-
- Valotteau C., Prystopiuk V., Pietrocola G., Rindi S., Peterle D., De Filippis V., Foster T.J., Speziale P., and Dufrêne Y.F. (2017). Single-Cell and Single-Molecule Analysis Unravels the Multifunctionality of the Staphylococcus aureus Collagen-Binding Protein Cna. ACS Nano 11, 2160–2170. 10.1021/ACSNANO.6B08404. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
