This is a preprint.
Metabolic regulation of RNA methylation by the m6A-reader IGF2BP3
- PMID: 39554138
- PMCID: PMC11565949
- DOI: 10.1101/2024.10.31.621399
Metabolic regulation of RNA methylation by the m6A-reader IGF2BP3
Update in
-
IGF2BP3 redirects glycolytic flux to promote one-carbon metabolism and RNA methylation.Cell Rep. 2025 Oct 28;44(10):116330. doi: 10.1016/j.celrep.2025.116330. Epub 2025 Sep 26. Cell Rep. 2025. PMID: 41015030 Free PMC article.
Abstract
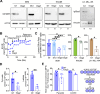
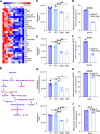
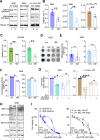
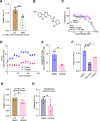
The interplay of RNA modifications - deposited by "writers", removed by "erasers" and identified by RNA binding proteins known as "readers" - forms the basis of the epitranscriptomic gene regulation hypothesis. Recent studies have identified the oncofetal RNA-binding protein IGF2BP3 as a "reader" of the N6-methyladenosine (m6A) modification and crucial for regulating gene expression. Yet, how its function as a reader overlaps with its critical oncogenic function in leukemia remains an open question. Here, we report the novel finding that the reader IGF2BP3 reprograms cellular metabolism, resulting in an altered ability of the "writers" to modify the epitranscriptome. In leukemia cells, IGF2BP3 supports increased glycolytic flux and one-carbon metabolism, leading to increased production of S-adenosyl methionine (SAM), a key substrate for methylation reactions within the cell. IGF2BP3 directly regulates the translation of MAT2B, the regulatory subunit of the methionine-adenosyltransferase complex, which is the final enzyme in a pathway leading to SAM production. This, in turn, results in increased m6A modifications on RNA, resulting in positive feedback regulation. This novel mechanism illustrates how metabolism mutually acts with epitranscriptomic modifications, underscoring the pervasive impact of IGF2BP3 in gene regulatory mechanisms governing a broad range of cancer-specific processes.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES DSR has served as a consultant to AbbVie, a pharmaceutical company that develops and markets drugs for hematologic disorders. DSR, MLT and AKJ are inventors on a patent application that includes the compound I3IN-002.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
