This is a preprint.
A trans-translation inhibitor is potentiated by zinc and kills Mycobacterium tuberculosis and non-tuberculous mycobacteria
- PMID: 39554143
- PMCID: PMC11566007
- DOI: 10.1101/2024.11.02.621434
A trans-translation inhibitor is potentiated by zinc and kills Mycobacterium tuberculosis and non-tuberculous mycobacteria
Update in
-
A trans-Translation Inhibitor is Potentiated by Zinc and Kills Mycobacterium tuberculosis and Nontuberculous Mycobacteria.ACS Infect Dis. 2025 May 9;11(5):1140-1152. doi: 10.1021/acsinfecdis.4c00963. Epub 2025 Apr 9. ACS Infect Dis. 2025. PMID: 40202906 Free PMC article.
Abstract
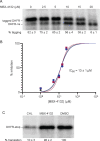
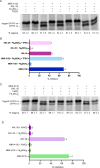
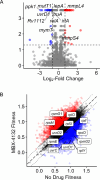
Mycobacterium tuberculosis poses a serious challenge for human health, and new antibiotics with novel targets are needed. Here we demonstrate that an acylaminooxadiazole, MBX-4132, specifically inhibits the trans-translation ribosome rescue pathway to kill M. tuberculosis. Our data demonstrate that MBX-4132 is bactericidal against multiple pathogenic mycobacterial species and kills M. tuberculosis in macrophages. We also show that acylaminooxadiazole activity is antagonized by iron but is potentiated by zinc. Our transcriptomic data reveals dysregulation of multiple metal homeostasis pathways after exposure to MBX-4132. Furthermore, we see differential expression of genes related to zinc sensing and efflux when trans-translation is inhibited. Taken together, these data suggest that there is a link between disturbing intracellular metal levels and acylaminooxadiazole-mediated inhibition of trans-translation. These findings provide an important proof-of-concept that trans-translation is a promising antitubercular drug target.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; metal homeostasis; non-tuberculous mycobacteria; oxadiazole; trans-translation.
Figures




References
-
- Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO
-
- Sekaggya-Wiltshire C., von Braun A., Scherrer A.U., Manabe Y.C., Buzibye A., Muller D., Ledergerber B., Gutteck U., Corti N., Kambugu A., et al. (2017). Anti-TB drug concentrations and drug-associated toxicities among TB/HIV-coinfected patients. Journal of Antimicrobial Chemotherapy 72, 1172–1177. 10.1093/jac/dkw534. - DOI - PMC - PubMed
-
- Chakaya J., Khan M., Ntoumi F., Aklillu E., Fatima R., Mwaba P., Kapata N., Mfinanga S., Hasnain S.E., Katoto P.D.M.C., et al. (2021). Global Tuberculosis Report 2020 – Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis 113, S7–S12. 10.1016/j.ijid.2021.02.107. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
