This is a preprint.
DNA nanodevice for analysis of force-activated protein extension and interactions
- PMID: 39554144
- PMCID: PMC11565787
- DOI: 10.1101/2024.10.25.620262
DNA nanodevice for analysis of force-activated protein extension and interactions
Update in
-
DNA nanodevice for analysis of force-activated protein extension and interactions.Nat Nanotechnol. 2025 Dec 15. doi: 10.1038/s41565-025-02086-w. Online ahead of print. Nat Nanotechnol. 2025. PMID: 41398070
Abstract
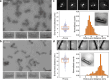
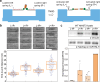
Force-induced changes in protein structure and function mediate cellular responses to mechanical stresses. Existing methods to study protein conformation under mechanical force are incompatible with biochemical and structural analysis. Taking advantage of DNA nanotechnology, including the well-defined geometry of DNA origami and programmable mechanics of DNA hairpins, we built a nanodevice to apply controlled forces to proteins. This device was used to study the R1-R2 segment of the talin1 rod domain as a model protein, which comprises two alpha-helical bundles that reversibly unfold under tension to expose binding sites for the cytoskeletal protein vinculin. Electron microscopy confirmed tension-dependent protein extension while biochemical analysis demonstrated enhanced vinculin binding under tension. The device could also be used in pull down assays with cell lysates, which identified filamins as novel tension-dependent talin binders. The DNA nanodevice is thus a valuable addition to the molecular toolbox for studying mechanosensitive proteins.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
