This is a preprint.
Elongation of the nascent avian foregut requires coordination of intrinsic and extrinsic cell behaviors
- PMID: 39554178
- PMCID: PMC11565921
- DOI: 10.1101/2024.10.31.621372
Elongation of the nascent avian foregut requires coordination of intrinsic and extrinsic cell behaviors
Update in
-
Elongation of the nascent avian foregut requires coordination of intrinsic and extrinsic cell behaviors.Dev Biol. 2025 Nov;527:277-288. doi: 10.1016/j.ydbio.2025.08.009. Epub 2025 Aug 12. Dev Biol. 2025. PMID: 40812618 Free PMC article.
Abstract
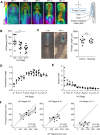
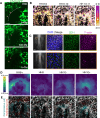
The foregut tube gives rise to the lungs and upper gastrointestinal tract, enabling vital functions of respiration and digestion. How the foregut tube forms during embryonic development has historically received considerable attention, but over the past few decades this question has primarily been addressed indirectly through studies on morphogenesis of the primitive heart tube, a closely related process. As a result, many aspects of foregut development remain unresolved. Here, we exploit the accessibility of the chick embryo to study the initial formation of the foregut tube, combining embryology with fate mapping, live imaging, and biomechanical analyses. The present study reveals that the foregut forms and elongates over a narrower time window than previously thought, and displays marked dorso-ventral and left-right asymmetries early in its development. Through tissue-specific ablation of endoderm along the anterior intestinal portal, we confirm its central biomechanical role in driving foregut morphogenesis, despite not directly contributing cells to the elongating tube. We further confirm the important role of this cell population in formation of the heart tube, with evidence that this role extends to later stages of cardiac looping as well. Together, these data reveal the need for an intricate balance between intrinsic cell behaviors and extrinsic forces for normal foregut elongation, and set the stage for future studies aimed at understanding the underlying molecular cues that coordinate this balance.
Figures



References
-
- Adelmann Howard B. 1922. “The Significance of the Prechordal Plate: An Interpretative Study.” American Journal of Anatomy 31 (1): 55–101. 10.1002/aja.1000310104. - DOI
-
- Aleksandrova Anastasiia, Andras Czirók Andras Szabó, Filla Michael B., Julius Hossain M., Whelan Paul F., Lansford Rusty, and Rongish Brenda J.. 2012. “Convective Tissue Movements Play a Major Role in Avian Endocardial Morphogenesis.” Developmental Biology 363 (2): 348–61. 10.1016/j.ydbio.2011.12.036. - DOI - PMC - PubMed
-
- Anderson Claire, Khan Mohsin A. F., Wong Frances, Solovieva Tatiana, Oliveira Nidia M. M., Baldock Richard A., Tickle Cheryll, Burt Dave W., and Stern Claudio D.. 2016. “A Strategy to Discover New Organizers Identifies a Putative Heart Organizer.” Nature Communications 7 (1): 12656. 10.1038/ncomms12656. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
