Exploring Hypertension: The Role of AT1 Receptors, Sartans, and Lipid Bilayers
- PMID: 39554401
- PMCID: PMC11561769
- DOI: 10.1021/acsomega.4c06351
Exploring Hypertension: The Role of AT1 Receptors, Sartans, and Lipid Bilayers
Abstract
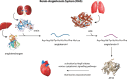
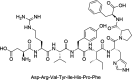
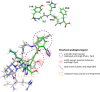
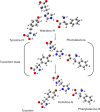
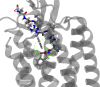
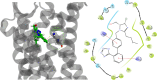
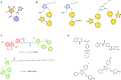
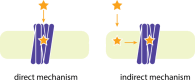
The rational design of AT1 receptor antagonists represents a pivotal approach in the development of therapeutic agents targeting cardiovascular pathophysiology. Sartans, a class of compounds engineered to inhibit the binding and activation of Angiotensin II on the AT1 receptor, have demonstrated significant clinical efficacy. This review explores the multifaceted role of sartans in mitigating hypertension and related complications. We highlight the integration of crystallography, computational simulations, and NMR spectroscopy to elucidate sartan-AT1 receptor interactions, providing a foundation for the next-generation antagonist design. The review also delves into the challenges posed by the high lipophilicity and suboptimal bioavailability of sartans, emphasizing advancements in nanotechnology and novel drug delivery systems. Additionally, we discuss the impact of lipid bilayers on the AT1 receptor conformation and drug binding, underscoring the importance of the lipidic environment in receptor-drug interactions. We suggest that optimizing drug design to account for these factors could enhance the therapeutic potential of AT1 receptor antagonists, paving the way for improved cardiovascular health outcomes.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
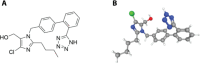
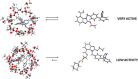
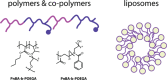
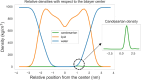

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
