IL-18 biology in severe asthma
- PMID: 39554494
- PMCID: PMC11566457
- DOI: 10.3389/fmed.2024.1486780
IL-18 biology in severe asthma
Erratum in
-
Corrigendum: IL-18 biology in severe asthma.Front Med (Lausanne). 2025 Jan 21;12:1558229. doi: 10.3389/fmed.2025.1558229. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39906600 Free PMC article.
Abstract
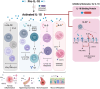
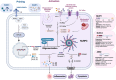
The role of interleukin-18 (IL-18) and inflammasomes in chronic inflammatory airway diseases, such as asthma and chronic obstructive pulmonary disease (COPD), has garnered significant attention in recent years. This review aims to provide an overview of the current understanding of IL-18 biology, the associated signaling pathways, and the involvement of inflammasome complexes in airway diseases. We explore the multifaceted role of IL-18 in asthma pathophysiology, including its interactions with other cytokines and contributions to both T2 and non-T2 inflammation. Importantly, emerging evidence highlights IL-18 as a critical player in severe asthma, contributing to chronic airway inflammation, airway hyperresponsiveness (AHR), and mucus impaction. Furthermore, we discuss the emerging evidence of IL-18's involvement in autoimmunity and highlight potential therapeutic targets within the IL-18 and inflammasome pathways in severe asthma patients with evidence of infections and airway autoimmune responses. By synthesizing recent advancements and ongoing research, this review underscores the importance of IL-18 as a potential novel therapeutic target in the treatment of severe asthma and other related conditions.
Keywords: IL-18; asthma; autoimmunity; eosinophilia; inflammasome.
Copyright © 2024 Thawanaphong, Nair, Volfson, Nair and Mukherjee.
Conflict of interest statement
MM reports research grants from Sanofi, Methapharm Specialty Pharamceuticals and Mirimus, consulting fees from AstraZeneca, Sanofi, Respiplus, GSK, Mirimus. PN reports research grants from AstraZeneca, Teva, Sanofi, Foresee, Roche, Genentech and consulting fees from AstraZeneca, Teva, Sanofi, GSK, Methapharm, Arrowhead pharma. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

