Integrating enzyme-nanoparticles bring new prospects for the diagnosis and treatment of immune dysregulation in periodontitis
- PMID: 39554809
- PMCID: PMC11564189
- DOI: 10.3389/fcimb.2024.1494651
Integrating enzyme-nanoparticles bring new prospects for the diagnosis and treatment of immune dysregulation in periodontitis
Abstract
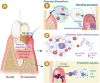
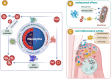
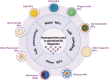
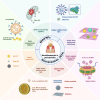
Enzymes play a significant role in mediating inflammatory and immune responses in periodontitis. Effective diagnosis, timely treatment, and continuous management of periodontal enzymes are essential to prevent undesirable consequences; however, this remains a significant challenge. Nanoparticles (NPs) have attracted significant attention in biomedicine because of their advantageous nanosized effects. NPs are conjugated with specific enzyme substrates at responsive sites that are triggered by periodontitis enzyme biomarkers, leading to functional or characteristic changes. In contrast, NPs with enzyme-mimetic activities exhibit catalytic activity, effectively destroying pathogenic biofilms and modulating the immune response in periodontitis. The unique properties of enzyme-targeting NPs have enabled the development of biosensors and fluorescent probes capable of identifying enzyme biomarkers associated with periodontitis. Enzyme-responsive and enzyme-mimetic NPs both exert therapeutic applications in the treatment of periodontitis. In this review, we provide a comprehensive overview of the enzymes associated with periodontitis, the mechanisms of enzyme-responsive and enzyme-mimetic NPs, recent advancements in the use of NPs for detecting these enzymes, and the therapeutic applications of NPs in targeting or mimicking enzyme functions. We also discuss the challenges and prospects of using NPs in the diagnosis and treatment of periodontitis.
Keywords: biosensors; enzyme-responsive nanoparticles; fluorescent probes; inflammatory; nanozymes; periodontitis biomarkers.
Copyright © 2024 Zhang, Wang, Shen, Wang, Cao, Deng, Meng and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Combined Ferromagnetic Nanoparticles for Effective Periodontal Biofilm Eradication in Rat Model.Int J Nanomedicine. 2023 May 9;18:2371-2388. doi: 10.2147/IJN.S402410. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37192894 Free PMC article.
-
Thermo-Responsive Hydrogel Containing Microfluidic Chitosan Nanoparticles Loaded with Opuntia ficus-indica Extract for Periodontitis Treatment.Int J Mol Sci. 2024 Aug 29;25(17):9374. doi: 10.3390/ijms25179374. Int J Mol Sci. 2024. PMID: 39273327 Free PMC article.
-
Nanozymes: From New Concepts, Mechanisms, and Standards to Applications.Acc Chem Res. 2019 Aug 20;52(8):2190-2200. doi: 10.1021/acs.accounts.9b00140. Epub 2019 Jul 5. Acc Chem Res. 2019. PMID: 31276379
-
Nanozyme-based electrochemical biosensors for disease biomarker detection.Analyst. 2020 Jul 7;145(13):4398-4420. doi: 10.1039/d0an00558d. Epub 2020 May 21. Analyst. 2020. PMID: 32436931 Review.
-
Spotlight on therapeutic efficiency of green synthesis metals and their oxide nanoparticles in periodontitis.J Nanobiotechnology. 2024 Jan 5;22(1):21. doi: 10.1186/s12951-023-02284-5. J Nanobiotechnology. 2024. PMID: 38183090 Free PMC article. Review.
Cited by
-
Nanozymes Empower Periodontitis Treatment: New Strategies and Clinical Application Prospects.Biomater Res. 2025 May 20;29:0210. doi: 10.34133/bmr.0210. eCollection 2025. Biomater Res. 2025. PMID: 40395276 Free PMC article. Review.
References
-
- Alhogail S., Suaifan G., Bizzarro S., Kaman W. E., Bikker F. J., Weber K., et al. . (2018). On site visual detection of Porphyromonas gingivalis related periodontitis by using a magnetic-nanobead based assay for gingipains protease biomarkers. Mikrochim. Acta 185, 149. doi: 10.1007/s00604-018-2677-x - DOI - PubMed
-
- Arias-Bujanda N., Regueira-Iglesias A., Balsa-Castro C., Nibali L., Donos N., Tomás I. (2019). Accuracy of single molecular biomarkers in gingival crevicular fluid for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 46, 1166–1182. doi: 10.1111/jcpe.13188 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

