Preventing acute neurotoxicity of CNS therapeutic oligonucleotides with the addition of Ca2+ and Mg2+ in the formulation
- PMID: 39554992
- PMCID: PMC11567125
- DOI: 10.1016/j.omtn.2024.102359
Preventing acute neurotoxicity of CNS therapeutic oligonucleotides with the addition of Ca2+ and Mg2+ in the formulation
Abstract
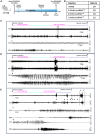
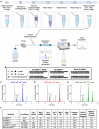
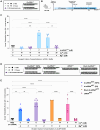
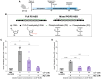
Oligonucleotide therapeutics (ASOs and siRNAs) have been explored for modulation of gene expression in the central nervous system (CNS), with several drugs approved and many in clinical evaluation. Administration of highly concentrated oligonucleotides to the CNS can induce acute neurotoxicity. We demonstrate that delivery of concentrated oligonucleotides to the CSF in awake mice induces acute toxicity, observable within seconds of injection. Electroencephalography and electromyography in awake mice demonstrated seizures. Using ion chromatography, we show that siRNAs can tightly bind Ca2+ and Mg2+ up to molar equivalents of the phosphodiester/phosphorothioate bonds independently of the structure or phosphorothioate content. Optimization of the formulation by adding high concentrations (above biological levels) of divalent cations (Ca2+ alone, Mg2+ alone, or Ca2+ and Mg2+) prevents seizures with no impact on the distribution or efficacy of the oligonucleotide. The data here establish the importance of adding Ca2+ and Mg2+ to the formulation for the safety of CNS administration of therapeutic oligonucleotides.
Keywords: CNS therapeutics; Huntington’s disease; MT: Oligonucleotides: Therapies and Applications; RNAi; brain delivery; genetic diseases; neurological disorders; oligonucleotide-based therapies; oligonucleotides.
© 2024 The Author(s).
Conflict of interest statement
A.K. and N.A. are co-founders, on the scientific advisory board, and hold equities of Atalanta Therapeutics. A.K. is a founder of Comanche Pharmaceuticals and on the scientific advisory board of Aldena Therapeutics, AlltRNA, Prime Medicine, and EVOX Therapeutics. N.A. is on the scientific advisory board of the Huntington’s Disease Society of America (HDSA). Select authors hold patents or on patent applications relating to the divalent siRNA and the methods described in this report.
Figures





Update of
-
Preventing acute neurotoxicity of CNS therapeutic oligonucleotides with the addition of Ca2+ and Mg2+ in the formulation.bioRxiv [Preprint]. 2024 Jun 8:2024.06.06.597639. doi: 10.1101/2024.06.06.597639. bioRxiv. 2024. Update in: Mol Ther Nucleic Acids. 2024 Oct 15;35(4):102359. doi: 10.1016/j.omtn.2024.102359. PMID: 38895198 Free PMC article. Updated. Preprint.
References
-
- Kuter D.J., Bonkovsky H.L., Monroy S., Ross G., Guillén-Navarro E., Cappellini M.D., Minder A.E., Hother-Nielsen O., Ventura P., Jia G., et al. Efficacy and safety of givosiran for acute hepatic porphyria: Final results of the randomized phase III ENVISION trial. J. Hepatol. 2023;79:1150–1158. doi: 10.1016/j.jhep.2023.06.013. - DOI - PubMed
-
- van Roon-Mom W., Ferguson C., Aartsma-Rus A. From Failure to Meet the Clinical Endpoint to U.S. Food and Drug Administration Approval: 15th Antisense Oligonucleotide Therapy Approved Qalsody (Tofersen) for Treatment of SOD1 Mutated Amyotrophic Lateral Sclerosis. Nucleic Acid Therapeut. 2023;33:234–237. doi: 10.1089/nat.2023.0027. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

