Acetate cellulose fibrous scaffold is suitable for cultivated fat production
- PMID: 39555016
- PMCID: PMC11564054
- DOI: 10.1016/j.crfs.2024.100903
Acetate cellulose fibrous scaffold is suitable for cultivated fat production
Abstract
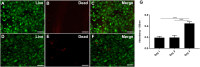
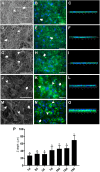
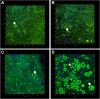
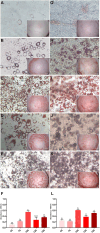
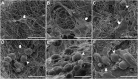
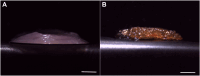
Fat is an essential component of meat which contributes to its sensory characteristics. Therefore, producing cultivated fat is essential to replicate the texture, flavor, and juiciness of conventional meat. One of the challenges in obtaining cultivated fat is that once adipocytes reach differentiation in culture, they tend to float. In this study, we tested whether immortalized pre-adipocytes could be viable, grow, and differentiate when cultivated onto a fibrous scaffold produced by the electrospun of cellulose acetate. Our results demonstrated that the cells attach, proliferate, colonize, and differentiate into mature adipocytes in the three-dimensional fibrous structure during the culture period. Moreover, when layers of the scaffold containing differentiated cells were stacked, it acquired a characteristic similar to conventional animal fat. Therefore, this research suggests that fibrous scaffolds produced using cellulose acetate are a promising substrate for producing cultivated fat.
Keywords: Adipocytes; Adipogenic differentiation; Cellulose acetate; Cultivated fat; Electrospinning; Scaffold.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Berry R., Fretz J.A., MacDougald O., Klibansky A., Rosen C.J., Rodeheffer M.S., et al. Osteoimmunology: Interactions of the Immune and Skeletal Systems. second ed. Elsevier Inc.; 2016. Marrow adipose tissue and its interactions with the skeletal, hematopoietic, and immune systems; pp. 345–352. - DOI
-
- César Farias de Queiroz J., Isabel Cardoso Alonso-Vale M., Curi R., Bessa Lima F. vol. 53. 2009. (Controle da adipogênese por ácidos graxos Control of adipogenesis by fatty acids). - PubMed
LinkOut - more resources
Full Text Sources

