ProC6C, a novel multi-stage malaria vaccine, elicits functional antibodies against the minor and central repeats of the Circumsporozoite Protein in human adults
- PMID: 39555079
- PMCID: PMC11563800
- DOI: 10.3389/fimmu.2024.1481829
ProC6C, a novel multi-stage malaria vaccine, elicits functional antibodies against the minor and central repeats of the Circumsporozoite Protein in human adults
Abstract
Introduction: ProC6C is a multi-stage malaria vaccine which includes Plasmodium falciparum Circumsporozoite Protein (PfCSP), Pfs48/45 and Pfs230 sequences, designed to elicit functional antibodies that prevent sporozoite invasion of human hepatocytes (PfCSP) and parasite development in mosquitoes (Pfs48/45 and Pfs230). ProC6C formulated on Alhydrogel was evaluated in combination with Matrix-M in a Phase 1 trial in Burkina Faso. The PfCSP antibody responses were assessed for magnitude, specificity, avidity and functionality. These results compliment the prior reported safety and tolerability of ProC6C as well as the transmission reducing activity of ProC6C.
Methods: The PfCSP response of ProC6C in Burkinabes in the Phase 1 trial (PACTR202201848463189) was profiled through the three vaccine administrations of 100 µg protein on Alhydrogel® alone (AlOH) or combined with 50 µg Matrix-M™ adjuvant (AlOH/Matrix-M). Serology was completed against full-length PfCSP and major/minor repeat peptides using antibody equivalence to PfCSP monoclonal antibodies (mAb 311, mAb 317 and mAb L9). Comparison of the ProC6C responses were made to those that received RTS,S/AS01 in a study conducted in Thailand. Bio-Layer Interferometry was further used to determine antibody avidity. The human IgG was subsequently purified, pooled, and evaluated in a mouse sporozoite challenge model to determine functionality.
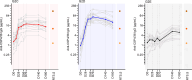
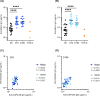
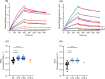
Results: A single administration of ProC6C-AlOH/Matrix-M seroconverted 19 of 20 volunteers against PfCSP and significantly enhanced antibody titers to major and minor repeats (and present through D180). At D70, ProC6C-AlOH/Matrix-M PfCSP antibodies were found to be similar to responder pools generated from Thai adults receiving RTS,S/AS01. Additionally, ProC6C antibodies were found to be competitive to established PfCSP antibodies such as mAb 317 and mAb L9. The purified and pooled IgG from human volunteers, used in a passive transfer mouse sporozoite challenge model, showed a median of 50% inhibition (P=0.0058). ProC6C PfCSP antibodies were functional in this in vivo assessment and consistent with inhibition seen by other Circumsporozoite vaccines in this model.
Discussion: This analysis supports continued investigation of the antibody responses elicited by the ProC6C multi-stage malaria vaccine. This Phase 1 clinical trial demonstrated the short PfCSP sequence included in ProC6C can induce significant PfCSP antibodies in humans, which importantly were determined to be functional.
Keywords: CSP; Circumsporozoite protein; Malaria; Matrix-M; antibodies; clinical trial; vaccine.
Copyright © 2024 Plieskatt, Ofori, Naghizadeh, Miura, Flores-Garcia, Borbye-Lorenzen, Tiono, Skogstrand, Sagara, Zavala and Theisen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Pendyala G, Calvo-Calle JM, Moreno A, Kane RS. A multivalent Plasmodium falciparum circumsporozoite protein-based nanoparticle malaria vaccine elicits a robust and durable antibody response against the junctional epitope and the major repeats. Bioeng Transl Med. (2023) 8:e10514. doi: 10.1002/btm2.10514 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

