Spatial-temporal regulation of the prostanoid receptor EP2 co-ordinates PGE2-mediated cAMP signaling in decidualizing human endometrium
- PMID: 39555416
- PMCID: PMC11567134
- DOI: 10.1016/j.isci.2024.111170
Spatial-temporal regulation of the prostanoid receptor EP2 co-ordinates PGE2-mediated cAMP signaling in decidualizing human endometrium
Abstract
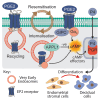
Decidualization denotes the differentiation of endometrial stromal cells into specialized decidual cells, essential for embryo implantation and pregnancy. The process requires coordination of progesterone and cAMP signaling, which converge on downstream transcription factors. PGE2 and relaxin, acting, respectively, through Gαs-coupled GPCRs EP2 and RXFP1, are putative candidates for generating cAMP in differentiating stromal cells. Here, we show that PGE2 is less efficacious than relaxin in elevating intracellular cAMP levels in primary stromal cells but more effective at driving the expression of decidual genes. PGE2-and relaxin-induced cAMP generation involves receptor internalization, but EP2 is endocytosed into very early endosomes (VEEs). Perturbation of VEE machinery through depletion of key trafficking proteins; APPL1 and GIPC, dysregulates PGE2-dependent cAMP profiles and disrupts key decidual signaling pathways, resulting in a disordered differentiation response. We demonstrate that regulation of EP2 via internalization is essential for coordinated activation of the downstream signaling cascades that govern decidualization.
Keywords: Endocrinology; Health sciences; Internal medicine; Medical specialty; Medicine; Reproductive medicine.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Single-cell analysis of prostaglandin E2-induced human decidual cell in vitro differentiation: a minimal ancestral deciduogenic signal†.Biol Reprod. 2022 Jan 13;106(1):155-172. doi: 10.1093/biolre/ioab183. Biol Reprod. 2022. PMID: 34591094 Free PMC article.
-
Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells.J Clin Endocrinol Metab. 2004 Jan;89(1):324-34. doi: 10.1210/jc.2003-030498. J Clin Endocrinol Metab. 2004. PMID: 14715868
-
Progesterone-dependent decidualization of the human endometrium is mediated by cAMP.Endocrine. 1997 Jun;6(3):301-7. doi: 10.1007/BF02820507. Endocrine. 1997. PMID: 9368687
-
Cyclic decidualization of the human endometrium in reproductive health and failure.Endocr Rev. 2014 Dec;35(6):851-905. doi: 10.1210/er.2014-1045. Epub 2014 Aug 20. Endocr Rev. 2014. PMID: 25141152 Review.
-
Implantation in the baboon: endometrial responses.Semin Reprod Endocrinol. 1999;17(3):257-65. doi: 10.1055/s-2007-1016233. Semin Reprod Endocrinol. 1999. PMID: 10797944 Review.
Cited by
-
Molecular Basis of Impaired Decidualization in the Eutopic Endometrium of Endometriosis Patients.Cells. 2025 Feb 21;14(5):326. doi: 10.3390/cells14050326. Cells. 2025. PMID: 40072055 Free PMC article. Review.
-
Prostaglandin E2 and Progesterone Receptor Coordinately Regulate Primary Cilia for Proper Decidualization.FASEB J. 2025 Aug 15;39(15):e70919. doi: 10.1096/fj.202500961RR. FASEB J. 2025. PMID: 40772309 Free PMC article.
-
Retromer Opposes Opioid-Induced Downregulation of the Mu Opioid Receptor.bioRxiv [Preprint]. 2024 Dec 2:2024.12.02.626482. doi: 10.1101/2024.12.02.626482. bioRxiv. 2024. PMID: 39677727 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources

