Muscle fibroblasts and stem cells stimulate motor neurons in an age and exercise-dependent manner
- PMID: 39555723
- PMCID: PMC11896526
- DOI: 10.1111/acel.14413
Muscle fibroblasts and stem cells stimulate motor neurons in an age and exercise-dependent manner
Abstract
Exercise preserves neuromuscular function in aging through unknown mechanisms. Skeletal muscle fibroblasts (FIB) and stem cells (MuSC) are abundant in skeletal muscle and reside close to neuromuscular junctions, but their relative roles in motor neuron maintenance remain undescribed. Using direct cocultures of embryonic rat motor neurons with either human MuSC or FIB, RNA sequencing revealed profound differential regulation of the motor neuron transcriptome, with FIB generally favoring neuron growth and cell migration and MuSC favoring production of ribosomes and translational machinery. Conditioned medium from FIB was superior to MuSC in preserving motor neurons and increasing their maturity. Lastly, we established the importance of donor age and exercise status and found an age-related distortion of motor neuron and muscle cell interaction that was fully mitigated by lifelong physical activity. In conclusion, we show that human muscle FIB and MuSC synergistically stimulate the growth and viability of motor neurons, which is further amplified by regular exercise.
Keywords: aging; neural plasticity; neurodegeneration; sarcopenia; satellite stem cell; skeletal muscle; training.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

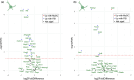

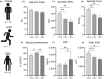

References
-
- Agley, C. C. , Rowlerson, A. M. , Velloso, C. P. , Lazarus, N. R. , & Harridge, S. D. R. (2013). Human skeletal muscle fibroblasts, but not myogenic cells, readily undergo adipogenic differentiation. Journal of Cell Science, 126, 5610–5625. - PubMed
-
- Arshadi, C. , Günther, U. , Eddison, M. , Harrington, K. I. S. , & Ferreira, T. A. (2021). SNT: A unifying toolbox for quantification of neuronal anatomy. Nature Methods, 18, 374–377. - PubMed
-
- Bechshøft, C. J. L. , Jensen, S. M. , Schjerling, P. , Andersen, J. L. , Svensson, R. B. , Eriksen, C. S. , Mkumbuzi, N. S. , Kjaer, M. , & Mackey, A. L. (2019). Age and prior exercise in vivo determine the subsequent in vitro molecular profile of myoblasts and nonmyogenic cells derived from human skeletal muscle. American Journal of Physiology‐Cell Physiology, 316, C898–C912. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

