Comprehensive Modular Synthesis of Ganglioside Glycans and Evaluation of their Binding Affinities to Siglec-7 and Siglec-9
- PMID: 39555730
- PMCID: PMC11727393
- DOI: 10.1002/advs.202412815
Comprehensive Modular Synthesis of Ganglioside Glycans and Evaluation of their Binding Affinities to Siglec-7 and Siglec-9
Abstract
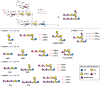
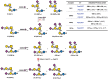
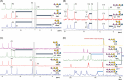
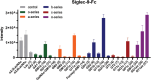
In the present work, bacterial glycosyltransferases are utilized to construct ganglioside glycans in a convergent approach via a sugar‒nucleotide regeneration system and one-pot multienzyme reactions. Starting from β-lactoside enables the diversification of both the glycan moieties and the linkages in the lower α-arm and upper β-arm. Overall, a comprehensive panel of 24 natural a-series (GM3, GM2, GM1a, GD1a, GT1a, and fucosyl-GM1), b-series (GD3, GD2, GD1b, GT1b, and GQ1b), c-series (GT3, GT2, GT1c, GQ1c, and GP1c), α-series (GM1α, GD1aα, and GT1aα), and o-series (GA2, GA1, GM1b, GalNAc-GM1b, and GD1c) ganglioside glycans are prepared, which are suitable for biological studies and further applications. Moreover, a microarray is constructed with these synthesized ganglioside glycans to investigate their binding specificity with recombinant Fc-fused Siglec-7 and Siglec-9, which are immune checkpoint-like glycan recognition proteins on natural killer cells. The microarray binding results reveal that GD3 and GT1aα are specific ligands for Siglec-7 and Siglec-9, respectively, and this discovery can lead to the identification of appropriate ligands for investigating the roles of these Siglecs in immunomodulation.
Keywords: Siglec; carbohydrates; chemoenzymatic synthesis; gangliosides; glycan microarray.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Simons K., Toomre D., Nat. Rev. Mol. Cell. Biol. 2000, 1, 31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
