Characteristic alterations of gut microbiota and serum metabolites in patients with chronic tinnitus: a multi-omics analysis
- PMID: 39555931
- PMCID: PMC11705945
- DOI: 10.1128/spectrum.01878-24
Characteristic alterations of gut microbiota and serum metabolites in patients with chronic tinnitus: a multi-omics analysis
Abstract
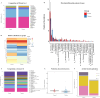
Chronic tinnitus is a central nervous system disorder. Currently, the effects of gut microbiota on tinnitus remain unexplored. To explore the connection between gut microbiota and tinnitus, we conducted 16S rRNA sequencing of fecal microbiota and serum metabolomic analysis in a cohort of 70 patients with tinnitus and 30 healthy volunteers. We used the weighted gene co-expression network method to analyze the relationship between the gut microbiota and the serum metabolites. The random forest technique was utilized to select metabolites and gut taxa to construct predictive models. A pronounced gut dysbiosis in the tinnitus group, characterized by reduced bacterial diversity, an increased Firmicutes/Bacteroidetes ratio, and some opportunistic bacteria including Aeromonas and Acinetobacter were enriched. In contrast, some beneficial gut probiotics decreased, including Lactobacillales and Lactobacillaceae. In serum metabolomic analysis, serum metabolic disturbances in tinnitus patients and these differential metabolites were enriched in pathways of neuroinflammation, neurotransmitter activity, and synaptic function. The predictive models exhibited great diagnostic performance, achieving 0.94 (95% CI: 0.85-0.98) and 0.96 (95% CI: 0.86-0.99) in the test set. Our study suggests that changes in gut microbiota could potentially influence the occurrence and chronicity of tinnitus, and exert regulatory effects through changes in serum metabolites. Overall, this research provides new perceptions into the potential role of gut microbiota and serum metabolite in the pathogenesis of tinnitus, and proposes the "gut-brain-ear" concept as a pathomechanism underlying tinnitus, with significant clinical diagnostic implications and therapeutic potential.IMPORTANCETinnitus affects millions of people worldwide. Severe cases may lead to sleep disorders, anxiety, and depression, subsequently impacting patients' lives and increasing societal healthcare expenditures. However, tinnitus mechanisms are poorly understood, and effective therapeutic interventions are currently lacking. We discovered the gut microbiota and serum metabolomics changes in patients with tinnitus, and provided the potential pathological mechanisms of dysregulated gut flora in chronic tinnitus. We proposed the innovative concept of the "gut-brain-ear axis," which underscores the exploration of gut microbiota impact on susceptibility to chronic tinnitus through serum metabolic profile modulation. We also reveal novel biomarkers associated with chronic tinnitus, offering a new conceptual framework for further investigations into the susceptibility of patients, potential treatment targets for tinnitus, and assessing patient prognosis. Subsequently, gut microbiota and serum metabolites can be used as molecular markers to assess the susceptibility and prognosis of tinnitus.Furthermore, fecal transplantation may be used to treat tinnitus.
Keywords: chronic tinnitus; gut microbiota; gut-brain-ear axis; multi-omics analysis; serum metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

