Rivaroxaban for 18 Months Versus 6 Months in Patients With Cancer and Acute Low-Risk Pulmonary Embolism: An Open-Label, Multicenter, Randomized Clinical Trial (ONCO PE Trial)
- PMID: 39556015
- PMCID: PMC11875411
- DOI: 10.1161/CIRCULATIONAHA.124.072758
Rivaroxaban for 18 Months Versus 6 Months in Patients With Cancer and Acute Low-Risk Pulmonary Embolism: An Open-Label, Multicenter, Randomized Clinical Trial (ONCO PE Trial)
Abstract
Background: The optimal duration of anticoagulation therapy for patients with cancer and acute low-risk pulmonary embolism (PE) is clinically relevant, but evidence is lacking. Prolonged anticoagulation therapy could have a potential benefit for prevention of thrombotic events; however, it could also increase the risk of bleeding.
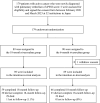
Methods: In a multicenter, open-label, adjudicator-blinded, randomized clinical trial at 32 institutions in Japan, we randomly assigned patients with cancer and acute low-risk PE of the simplified version of the Pulmonary Embolism Severity Index score of 1, in a 1:1 ratio, to receive either an 18-month or a 6-month rivaroxaban treatment. The primary end point was recurrent venous thromboembolism (VTE) at 18 months. The major secondary end point was major bleeding at 18 months according to the criteria of the International Society on Thrombosis and Hemostasis. The primary hypothesis was that an 18-month treatment was superior to a 6-month treatment in terms of the primary end point.
Results: From February 2021 to March 2023, 179 patients were randomized, and after the exclusion of one patient who withdrew consent, 178 were included in the intention-to-treat population: 89 patients in the 18-month rivaroxaban group and 89 in the 6-month rivaroxaban group. The mean age was 65.7 years; 47% of the patients were men, and 12% had symptoms of PE at baseline. The primary end point of recurrent VTE occurred in 5 of the 89 patients (5.6%) in the 18-month rivaroxaban group and in 17 of the 89 (19.1%) in the 6-month rivaroxaban group (odds ratio, 0.25 [95% CI, 0.09-0.72]; P=0.01). Among 22 recurrent VTE, 5 patients presented with a symptomatic recurrent VTE; recurrent PE occurred in 11 patients, including 2 with main and 4 with lobar PEs; and recurrent deep vein thrombosis was seen in 11 patients, including 3 with proximal deep vein thromboses. The major secondary end point of major bleeding occurred in 7 of the 89 patients (7.8%) in the 18-month rivaroxaban group and in 5 of the 89 patients (5.6%) in the 6-month rivaroxaban group (odds ratio, 1.43 [95% CI, 0.44-4.70]; P=0.55).
Conclusions: In patients with cancer and acute low-risk PE of the simplified version of the Pulmonary Embolism Severity Index score of 1, the 18-month rivaroxaban treatment was superior to the 6-month rivaroxaban treatment with respect to recurrent VTE events.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04724460.
Keywords: anticoagulants; cardio-oncology; neoplasms; pulmonary embolism; recurrence; rivaroxaban.
Conflict of interest statement
Dr Yamashita received lecture fees from Bayer Yakuhin, Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo and grant support from Bayer Yakuhin and Daiichi-Sankyo. Dr Morimoto reports lecture fees from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Pfizer, Tsumura, and UCB; manuscript fee from Pfizer; and advisory board fees from GlaxoSmithKline, Novartis, and Teijin. Dr Shibata received lecture fees from Novartis Pharmaceuticals KK and Otsuka Pharmaceuticals Co Ltd. Dr Nishimoto received lecture fees from Bayer Yakuhin, Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo. Dr Ogihara received lecture fees from Bayer Yakuhin, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo and research funds from Bayer Yakuhin and Daiichi Sankyo. Dr Ikeda received lecture fees from Bayer Yakuhin, Bristol-Myers Squibb, and Daiichi Sankyo. The other authors report no conflicts.
Figures




References
-
- Lyon AR, Lopez-Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. ; ESC Scientific Document Group. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244 - PubMed
-
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715 - PubMed
-
- Di Nisio M, Lee AY, Carrier M, Liebman HA, Khorana AA; Subcommittee on Haemostasis and Malignancy. Diagnosis and treatment of incidental venous thromboembolism in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13:880–883. doi: 10.1111/jth.12883 - PubMed
-
- Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376:2032–2039. doi: 10.1016/S0140-6736(10)60962-2 - PubMed
-
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, et al. ; ESC Scientific Document Group. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405 - PubMed

