Cyclin-dependent kinase 13 is indispensable for normal mouse heart development
- PMID: 39556044
- PMCID: PMC11911135
- DOI: 10.1111/joa.14175
Cyclin-dependent kinase 13 is indispensable for normal mouse heart development
Abstract
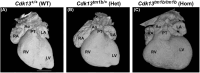
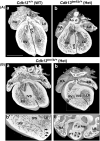
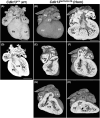
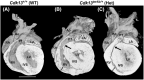
Congenital heart disease (CHD) has an incidence of approximately 1%. Over the last decade, sequencing studies including large cohorts of individuals with CHD have begun to unravel the genetic mechanisms underpinning CHD. This includes the identification of variants in cyclin-dependent kinase 13 (CDK13), in individuals with syndromic CHD. CDK13 encodes a serine/threonine protein kinase. The cyclin partner of CDK13 is cyclin K; this complex is thought to be important in transcription and RNA processing. Pathogenic variants in CDK13 cause CDK13-related disorder in humans, characterised by intellectual disability and developmental delay, recognisable facial features, feeding difficulties and structural brain defects, with 35% of individuals having CHD. To obtain a greater understanding for the role that this essential protein kinase plays in embryonic heart development, we have analysed a presumed loss of function Cdk13 transgenic mouse model (Cdk13tm1b). The homozygous mutants were embryonically lethal in most cases by E15.5. X-gal staining showed Cdk13 expression localised to developing facial regions, heart and surrounding areas at E10.5, whereas at E12.5, it was more widely present. In the E15.5 heart, staining was seen throughout. RT-qPCR showed significant reduction in Cdk13 transcript expression in homozygous compared with WT and heterozygous hearts at E10.5 and E12.5. Detailed morphological 3D analysis of embryonic and postnatal hearts was performed using high-resolution episcopic microscopy, which affords a more detailed analysis of structures such as cardiac valve leaflets and endocardial cushions, compared with more traditional histological techniques. We show that both the homozygous and heterozygous Cdk13tm1b mutants exhibit a range of CHD, including ventricular septal defects, bicuspid aortic valve, double outlet right ventricle and atrioventricular septal defects. 100% (n = 4) of homozygous hearts displayed CHD. Differential expression was seen in Cdk13tm1b homozygous mutants for two genes known to be necessary for normal heart development. The types of defects, and the presence of CHD in heterozygous mice (17.02%, n = 8/47), are consistent with the CDK13-related disorder phenotype in humans. This study provides important insights into the effects of reduced function of CDK13 in the mouse heart and contributes to our understanding of the mechanism behind this disorder as a cause of CHD.
Keywords: Cdk13; CHD; congenital heart disease; congenital heart disorders; cyclin‐dependant kinase 13; high‐resolution episcopic microscopy.
© 2024 The Author(s). Journal of Anatomy published by John Wiley & Sons Ltd on behalf of Anatomical Society.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures


References
-
- Anderson, R.H. , Mohun, T.J. , Spicer, D.E. , Bamforth, S.D. , Brown, N.A. , Chaudhry, B. et al. (2014) Myths and realities relating to development of the arterial valves. Journal of Cardiovascular Development and Disease, 1, 177–200.
-
- Anderson, R.H. , Wessels, A. & Vettukattil, J.J. (2010) Morphology and morphogenesis of atrioventricular septal defect with common atrioventricular junction. World J Pediatr Congenit Heart Surg, 1, 59–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

