Mavacamten in Patients With Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 128 Results From VALOR-HCM
- PMID: 39556124
- PMCID: PMC12063683
- DOI: 10.1161/CIRCULATIONAHA.124.072445
Mavacamten in Patients With Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 128 Results From VALOR-HCM
Erratum in
-
Correction to: Mavacamten in Patients With Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 128 Results from VALOR-HCM.Circulation. 2025 May 13;151(19):e1001. doi: 10.1161/CIR.0000000000001339. Epub 2025 May 12. Circulation. 2025. PMID: 40354453 Free PMC article. No abstract available.
Abstract
Background: In severely symptomatic patients with obstructive hypertrophic cardiomyopathy (HCM), VALOR-HCM trial (Study to Evaluate Mavacamten in Adults With Symptomatic Obstructive HCM Who Are Eligible for Septal Reduction Therapy [URL: https://clinicaltrials.gov; Unique identifier: NCT04349072]) reported that mavacamten reduced the short-term need for septal reduction therapy (SRT). The current report examined the longer-term effect of mavacamten through end of treatment at week 128.
Methods: A double-blind randomized placebo-controlled multicenter trial at 19 sites in the United States included symptomatic obstructive HCM patients referred for SRT (enrollment July 2020 through October 2021). The group initially randomized to mavacamten continued the drug for 128 weeks and the placebo to mavacamten group from week 16 to 128 (112-week exposure). Dose titrations were performed using echocardiographic left ventricular outflow tract gradient and left ventricular ejection fraction measurements. The principal end point was proportion of patients proceeding with SRT or remaining guideline-eligible at week 128.
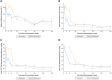
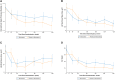
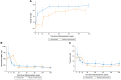
Results: At week 128, 17 of 108 (15.7%) patients in the total study sample met the composite end point (7 underwent SRT, 1 was SRT-eligible, and 9 SRT-status unevaluable). Additionally, 87 of 108 (80.5%) patients demonstrated ≥1 New York Heart Association class improvement by week 128, and 52 of 108 (48.1%) demonstrated ≥2, with a sustained reduction in resting and Valsalva left ventricular outflow tract gradients of 38.2 mm Hg and 59.4 mm Hg, respectively. Ninety-five of 108 (88%) patients transitioned to commercial mavacamten. Overall, 15 of 108 (13.8%) patients (5.41 per 100 patient-years) had an left ventricular ejection fraction <50% (2 with left ventricular ejection fraction ≤30%; 1 death). Of these, 12 of 15 (80%) continued treatment. New-onset atrial fibrillation occurred in 11 (10.2%) patients (4.55 per 100 patient-years).
Conclusions: In severely symptomatic obstructive HCM patients, sustained freedom from SRT was observed at 128 weeks, with nearly 90% patients remaining on long-term mavacamten.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04349072.
Keywords: cardiomyopathy, hypertrophic; mavacamten; septal reduction.
Conflict of interest statement
M. Desai reports consulting for Bristol Myers Squibb, Cytokinetics, Tenaya, and Medtronic; and research support to Cleveland Clinic from Bristol Myers Squibb, Cytokinetics, and Tenaya. A. Owens reports consulting for Bristol Myers Squibb, Cytokinetics, Pfizer, Biomarin, Tenaya, Lexicon, Stealth, Edgewise, and Renovacor; and receives grant support for research from Bristol Myers Squibb. Dr Saberi reports consulting for Bristol Myers Squibb and Cytokinetics. Dr Lakdawala has received consulting incomes from Bristol Myers Squibb, Pfizer, Tenaya, Cytokinetics and Akros and research support from Bristol Myers Squibb and Pfizer. Drs Naidu, Wang, Sherrid, and Tower-Rader report consulting for Bristol Myers Squibb and Cytokinetics. Drs Smedira and Geske report consultation with Bristol Myers Squibb. Dr Fermin reports conflicts from Bristol Myers Squibb (consulting, speaking), Pfizer (consulting), BridgeBio (consulting, speaking). Drs Nissen and Wolski work for C5 Research and are employees of Cleveland Clinic, which received payments for the present research from Bristol Myers Squibb. Drs Gong, Mudarris, Sehnert, and Lampl are employees of Bristol Myers Squibb. Dr Cremer was employed during the conduct of the trial by Cleveland Clinic, which received payments for current research from Bristol Myers Squibb. The other authors report no conflicts.
Figures

References
-
- Maron BJ, Desai MY, Nishimura RA, Spirito P, Rakowski H, Towbin JA, Dearani JA, Rowin EJ, Maron MS, Sherrid MV. Management of hypertrophic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:390–414. doi: 10.1016/j.jacc.2021.11.021 - PubMed
-
- Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales-Villa R, Basso C, Bezzina CR, Biagini E, Blom NA, de Boer RA, et al. ; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44:3503–3626. doi: 10.1093/eurheartj/ehad194 - PubMed
-
- Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, Day SM, Dearani JA, Epps KC, Evanovich L, Ferrari VA, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1239–e1311. doi: 10.1161/CIR.0000000000001250 - PubMed
-
- Braunwald E, Lambrew CT, Rockoff SD, Ross J, Jr, Morrow AG. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation. 1964;29:3–119. doi: 10.1161/01.cir.29.5s4.iv-3 - PubMed
-
- Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

