Diacerein ameliorates thioacetamide-induced hepatic encephalopathy in rats via modulation of TLR4/AQP4/MMP-9 axis
- PMID: 39556255
- PMCID: PMC11573817
- DOI: 10.1007/s11011-024-01457-x
Diacerein ameliorates thioacetamide-induced hepatic encephalopathy in rats via modulation of TLR4/AQP4/MMP-9 axis
Abstract
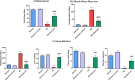
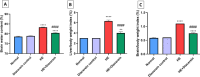
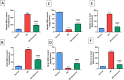
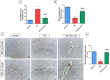
Astrocyte swelling, blood brain barrier (BBB) dissipation and the subsequent brain edema are serious consequences of persistent hyperammonemia in hepatic encephalopathy (HE) in which if inadequately controlled it will lead to brain death. The current study highlights the potential neuroprotective effect of diacerein against thioacetamide (TAA)-induced HE in acute liver failure rat model. HE was induced in male Sprague-Dawley rats via I.P. injection of TAA (200 mg/kg) for three alternative times/week at 3rd week of the experiment. Diacerein (50 mg/kg) was gavaged for 14 days prior to induction of HE and for further 7 days together with TAA injection for an overall period of 21 days. Diacerein attenuated TAA-induced HE in acute liver failure rat model; as proofed by significant lowering of serum and brain ammonia concentrations, serum AST and ALT activities and significant attenuation of both brain and hepatic MDA contents and IL-1β with marked increases in GSH contents (P < 0.0001). The neuroprotective effect of diacerein was demonstrated by marked improvement of motor and cognitive deficits, brain histopathological changes; hallmarks of HE. As shown by immunohistochemical results, diacerein markedly downregulated brain TLR4 expression which in turn significantly increased the GFAP expression, and significantly decreased AQP4 expression; the astrocytes swelling biomarkers (P < 0.0001). Moreover, diacerein preserved BBB integrity via downregulation of MMP-9 mediated digestion of tight junction proteins such as occludin (P < 0.0001). Collectively, diacerein ameliorated cerebral edema and maintained BBB integrity via modulation of TLR4/AQP4/MMP-9 axis thus may decrease the progression of HE induced in acute liver failure.
Keywords: Aquaporin 4; Diacerein; GFAP; Hepatic encephalopathy; MMP-9; TLR4.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
-
- Abd-ellatif RN, Hegab II, Atef MM, Sadek MT, Hafez YM (2019) Diacerein protects against glycerol-induced acute kidney injury: modulating oxidative stress, inflammation, apoptosis and necroptosis. Chemico-Biol Interact 306:47–53 - PubMed
-
- Abdelaziz RR, Elkashef WF, Said E (2015) Tranilast reduces serum IL-6 and IL-13 and protects against thioacetamide-induced acute liver injury and hepatic encephalopathy. Environ Toxicol Pharmacol 40:259–267 - PubMed
-
- Ali MHH, Messiha BAS, Abdel-Latif HA-T (2016) Protective effect of ursodeoxycholic acid, resveratrol, and N-acetylcysteine on nonalcoholic fatty liver disease in rats. Pharm Biol 54:1198–1208 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

