Amyloid-Related Imaging Abnormalities (ARIA) in Clinical Trials of Gantenerumab in Early Alzheimer Disease
- PMID: 39556389
- PMCID: PMC11574721
- DOI: 10.1001/jamaneurol.2024.3937
Amyloid-Related Imaging Abnormalities (ARIA) in Clinical Trials of Gantenerumab in Early Alzheimer Disease
Erratum in
-
Correction to Acknowledge Nonauthor Collaborators and Remove Potentially Identifiable Patient Information.JAMA Neurol. 2025 Feb 24;82(4):423. doi: 10.1001/jamaneurol.2025.0076. Online ahead of print. JAMA Neurol. 2025. PMID: 39992631 Free PMC article. No abstract available.
Abstract
Importance: Data from 2 phase 3 studies of gantenerumab, GRADUATE I/II, and their open-label extensions represent a resource to further characterize amyloid-related imaging abnormalities (ARIA), including long-term sequelae.
Objectives: To describe the characteristics of ARIA and risk factors and clinical consequences of ARIA-edema (ARIA-E).
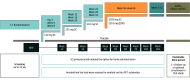
Design, setting, and participants: Secondary data collection from the GRADUATE I/II phase 3 randomized, double-blind, placebo-controlled, 116-week parallel-group studies and their open-label extensions, including PostGraduate, with up to 210 (mean, 125) weeks of total gantenerumab treatment were conducted between 2018 and 2023. The study included multicenter trials at 288 sites across 30 countries. GRADUATE I/II enrolled 985 and 980 participants, respectively, with early symptomatic Alzheimer disease (AD) and amyloid-beta (Aβ) pathology who were aged 50 to 90 years. PostGraduate enrolled 1382 participants (671 previously randomized to gantenerumab). Data were analyzed from November 2, 2022, to October 10, 2023.
Interventions: GRADUATE I/II participants were randomized 1:1 to gantenerumab or placebo. Nine-month uptitration was used to mitigate ARIA risk.
Main outcomes and measures: Postbaseline safety monitoring, including brain magnetic resonance imaging (MRI) findings, and adverse events and cognitive assessments.
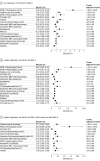
Results: The safety-evaluable MRI population of GRADUATE I/II comprised 1939 participants (mean age, 71.7 years; 1105 female [57.0%]). Severity of AD-related Aβ neuropathology (lower cerebrospinal fluid [CSF] Aβ42, hazard ratio [HR] for CSF Aβ42: 0.4; 95% CI, 0.2-0.7) and comorbid cerebrovascular pathology (Fazekas score: HR, 1.6; 95% CI, 1.3-2.0; total superficial siderosis count: HR, 1.9; 95% CI, 1.3-2.6; total microhemorrhage count: HR, 1.3; 95% CI, 1.0-1.5) may be important baseline risk factors for ARIA-E, in addition to apolipoprotein E (APOE) ε4 status (APOE ε4 heterozygous carrier: HR, 2.0; 95% CI, 1.4-2.8 and APOE ε4 homozygous carrier: HR, 4.7; 95% CI, 3.2-6.7). At the group level, ARIA-E did not impact long-term cognitive and functional performance (relative difference in adjusted means for Clinical Dementia Rating-Sum of Boxes was -9% in pooled GRADUATE analysis at week 116 and when censored at first ARIA-E). While taking gantenerumab, ARIA-E and ARIA-hemosiderin occurred in 24.9% (247 of 993) and 22.9% (227 of 993) participants, respectively; first ARIA-E occurred by week 64 in 86.2% (213 of 247) of participants with ARIA-E. Narratives are provided for all serious symptomatic ARIA-E cases.
Conclusions and relevance: These results show that in addition to APOE ε4 allele count, severity of Aβ neuropathology and comorbid cerebrovascular pathology may be relevant for clinicians prescribing anti-Aβ monoclonal antibodies for early AD and developing individualized safety monitoring plans. Evaluation of these risk factors in other anti-Aβ monoclonal antibodies is recommended.
Trial registrations: ClinicalTrials.gov Identifiers: NCT03444870, NCT03443973, NCT04374253.
Conflict of interest statement
Figures

References
-
- Eisai . LEQEMBI prescribing information. Accessed October 15, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269Orig1s00...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

