A scale-invariant log-normal droplet size distribution below the critical concentration for protein phase separation
- PMID: 39556435
- PMCID: PMC11573347
- DOI: 10.7554/eLife.94214
A scale-invariant log-normal droplet size distribution below the critical concentration for protein phase separation
Abstract
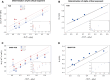
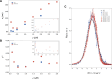
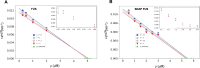
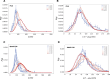
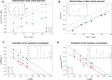
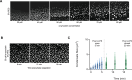
Many proteins have been recently shown to undergo a process of phase separation that leads to the formation of biomolecular condensates. Intriguingly, it has been observed that some of these proteins form dense droplets of sizeable dimensions already below the critical concentration, which is the concentration at which phase separation occurs. To understand this phenomenon, which is not readily compatible with classical nucleation theory, we investigated the properties of the droplet size distributions as a function of protein concentration. We found that these distributions can be described by a scale-invariant log-normal function with an average that increases progressively as the concentration approaches the critical concentration from below. The results of this scaling analysis suggest the existence of a universal behaviour independent of the sequences and structures of the proteins undergoing phase separation. While we refrain from proposing a theoretical model here, we suggest that any model of protein phase separation should predict the scaling exponents that we reported here from the fitting of experimental measurements of droplet size distributions. Furthermore, based on these observations, we show that it is possible to use the scale invariance to estimate the critical concentration for protein phase separation.
Keywords: critical exponents; none; phase transitions; physics of living systems; protein condensation; universal behaviour.
© 2024, Amico et al.
Conflict of interest statement
TA, SD, AL, MB, AT, MV, MF, AM No competing interests declared
Figures
Update of
- doi: 10.1101/2023.04.11.536478
- doi: 10.7554/eLife.94214.1
- doi: 10.7554/eLife.94214.2
References
-
- Bergmann RB, Bill A. On the origin of logarithmic-normal distributions: an analytical derivation, and its application to nucleation and growth processes. Journal of Crystal Growth. 2008;310:3135–3138. doi: 10.1016/j.jcrysgro.2008.03.034. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

