Ultra-Long-Term Anti-Inflammatory Polyphenol Capsule to Remodel the Microenvironment for Accelerating Osteoarthritis Healing by Single Dosage
- PMID: 39556697
- PMCID: PMC11672291
- DOI: 10.1002/advs.202407425
Ultra-Long-Term Anti-Inflammatory Polyphenol Capsule to Remodel the Microenvironment for Accelerating Osteoarthritis Healing by Single Dosage
Abstract
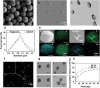
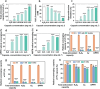
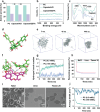
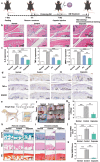
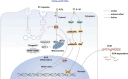
Osteoarthritis (OA) is a common chronic inflammatory disease that leads to disability and death. Existing therapeutic agents often require frequent use, which can lead to drug resistance and long-term side effects. Polyphenols have anti-inflammatory and antioxidant potential. However, they are limited by their short half-life and low bioavailability. This work presents a novel pure polyphenol capsule for sustained release of polyphenols, which is self-assembled via hydrophobic and hydrogen bonds. The capsule enhances cellular uptake, scavenges reactive oxygen and nitrogen species, reduces inflammatory markers, and remodels the OA microenvironment by inhibiting the p38 MAPK pathway. The capsule overcomes the limitations of short half-life and low bioavailability of polyphenols and achieves single-dose cure in mouse and dog OA models, providing an optimal therapeutic window for OA repair. Taking advantage of simple manufacturing, convenient administration, and pure polyphenol composition, these capsules show great potential for clinical treatment of osteoarthritis and chronic inflammatory diseases.
Keywords: antioxidant; capsules; osteoarthritis; polyphenol; single‐dose cure.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Glyn‐Jones S., Palmer A. J., Agricola R., Price A. J., Vincent T. L., Weinans H., Carr A. J., Lancet 2015, 386, 376. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
