Effects of Tirzepatide on the Clinical Trajectory of Patients With Heart Failure, Preserved Ejection Fraction, and Obesity
- PMID: 39556714
- PMCID: PMC11893002
- DOI: 10.1161/CIRCULATIONAHA.124.072679
Effects of Tirzepatide on the Clinical Trajectory of Patients With Heart Failure, Preserved Ejection Fraction, and Obesity
Abstract
Background: Patients with heart failure with preserved ejection fraction and obesity have significant disability and frequent exacerbations of heart failure. We hypothesized that tirzepatide, a long-acting agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptors, would improve a comprehensive suite of clinical end points, including measures of health status, functional capacity, quality of life, exercise tolerance, patient well-being, and medication burden, in these patients.
Methods: We randomized (double-blind) 731 patients with class II to IV heart failure, ejection fraction ≥50%, and body mass index ≥30 kg/m2 to tirzepatide (titrated up to 15 mg SC weekly; n=364) or placebo (n=367) added to background therapy for a median of 104 weeks (quartile 1, 66; quartile 3, 126 weeks). The primary end points were whether tirzepatide reduced the combined risk of cardiovascular death or worsening heart failure and improved Kansas City Cardiomyopathy Questionnaire Clinical Summary Score. The current expanded analysis included sensitivity analyses of the primary end points, 6-minute walk distance, EQ-5D-5L health state index, Patient Global Impression of Severity Overall Health score, New York Heart Association class, use of heart failure medications, and a hierarchical composite based on all-cause death, worsening heart failure, and 52-week changes in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score and 6-minute walk distance.
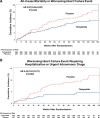
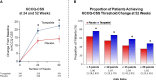
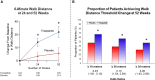
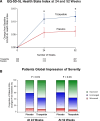
Results: Patients were 65.2±10.7 years of age; 53.8% (n=393) were female; body mass index was 38.2±6.7 kg/m2; Kansas City Cardiomyopathy Questionnaire Clinical Summary Score was 53.5±18.5; 6-minute walk distance was 302.8±81.7 m; and 53% (n=388) had a worsening heart failure event in the previous 12 months. Compared with placebo, tirzepatide produced a consistent beneficial effect across all composites of death and worsening heart failure events, analyzed as time to first event (hazard ratios, 0.41-0.67). At 52 weeks, tirzepatide increased the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score by 6.9 points (95% CI, 3.3-10.6; P<0.001), 6-minute walk distance 18.3 meters (95% CI, 9.9-26.7; P<0.001), and EQ-5D-5L 0.06 (95% CI, 0.03-0.09; P<0.001). The tirzepatide group shifted to a more favorable Patient Global Impression of Severity Overall Health score (proportional odds ratio, 1.99 [95% CI, 1.44-2.76]) and New York Heart Association class (proportional odds ratio, 2.26 [95% CI, 1.54-3.31]; both P<0.001) and required fewer heart failure medications (P=0.015). The broad spectrum of effects was reflected in benefits on the hierarchical composite (win ratio, 1.63 [95% CI, 1.17-2.28]; P=0.004).
Conclusions: Tirzepatide produced a comprehensive, meaningful improvement in heart failure across multiple complementary domains; enhanced health status, quality of life, functional capacity, exercise tolerance, and well-being; and reduced symptoms and medication burden in patients with heart failure with preserved ejection fraction and obesity.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04847557.
Keywords: heart failure, preserved ejection fraction; obesity; tirzepatide.
Conflict of interest statement
Dr Zile receives research support from the Department of Veterans Affairs (projects BX005943 and BX005848) and serves as a consultant for Abbott, Adona Medical, Aria CV, Avery Therapeutics, Inc, Boehringer-Ingelheim, Boston Scientific, Cardiovascular Research Foundation Clinical Trials Center, CVRx, DIASTOL Therapeutics, LLC, EBR, Edwards, Lilly, GenKardia, Innoventric, KestraMedical, Medtronic, Merck, Morphic Therapeutics, Novartis, Pulnova, Salubris Biotherapeutics, Sonata, SRNALYTICS, INC, V-WAVE, and Vectorious. Dr Borlaug receives research support from the National Institutes of Health (R01 HL128526, R01 HL162828, and U01 HL160226 from the National Heart, Lung, and Blood Institute) and the US Department of Defense, as well as research grant funding from AstraZeneca, Axon, Corvia, Novo Nordisk, and Tenax Therapeutics. Dr Borlaug has served as a consultant for Actelion, Amgen, Aria, Axon Therapies, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Lilly, Imbria, Janssen, Merck, Novo Nordisk, NGM, NXT, and VADovations. Dr Borlaug is named inventor (US Patent No. 10 307 179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure. Dr Kramer has served as a consultant for and received research grants from Lilly and has received research grants from Cytokinetics and Bristol Meyers Squibb. Dr Baum has served as consultant for Altimmune, Amgen, Beren Therapeutics, Boehringer Ingelheim, Lilly, Esperion, Ionis Pharmaceuticals, Madrigal Pharmaceuticals, Merck, Novartis, and Regeneron. Dr Litwin reports being on the patient selection committee for Corvia and Axon and being a consultant for Novo Nordisk and Lilly. Dr Menon reports no conflicts. Drs Ou, Weerakkody, Hurt, Kanu, and Murakami are employed by Eli Lilly and Company. Dr Packer has served as a consultant for 89bio, Abbvie, Actavis, Altimmune, Alnylam, Amarin, Amgen, Ardelyx, ARMGO, AstraZeneca, Attralus, Biopeutics, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Lilly, Imara, Medtronic, Moderna, Novartis, Pharmacocosmos, Reata, Regeneron, Roche, and Salamandra.
Figures


Comment in
-
Holistic View of Tirzepatide's Role in Obesity-Related HFpEF.Circulation. 2025 Mar 11;151(10):669-671. doi: 10.1161/CIRCULATIONAHA.125.073439. Epub 2025 Mar 10. Circulation. 2025. PMID: 40063724 No abstract available.
References
-
- Redfield MM, Borlaug BA. Heart failure with preserved ejection fraction: a review. JAMA. 2023;329:827–838. doi: 10.1001/jama.2023.2020 - PubMed
-
- Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049 - PubMed
-
- Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. ; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 - PubMed
-
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 - PubMed
-
- Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. ; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

