In vivo photoreceptor base editing ameliorates rhodopsin-E150K autosomal-recessive retinitis pigmentosa in mice
- PMID: 39556729
- PMCID: PMC11621631
- DOI: 10.1073/pnas.2416827121
In vivo photoreceptor base editing ameliorates rhodopsin-E150K autosomal-recessive retinitis pigmentosa in mice
Abstract
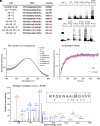
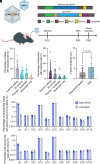
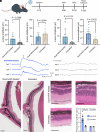
Rhodopsin, the prototypical class-A G-protein coupled receptor, is a highly sensitive receptor for light that enables phototransduction in rod photoreceptors. Rhodopsin plays not only a sensory role but also a structural role as a major component of the rod outer segment disc, comprising over 90% of the protein content of the disc membrane. Mutations in RHO which lead to structural or functional abnormalities, including the autosomal recessive E150K mutation, result in rod dysfunction and death. Therefore, correction of deleterious rhodopsin mutations could rescue inherited retinal degeneration, as demonstrated for other visual genes such as RPE65 and PDE6B. In this study, we describe a CRISPR/Cas9 adenine base editing strategy to correct the E150K mutation and demonstrate precise in vivo editing in a Rho-E150K mouse model of autosomal recessive retinitis pigmentosa (RP). Using ultraviolet-visible spectroscopy, mass spectrometry, and the G-protein activation assay, we characterized wild-type rhodopsin and rhodopsin variants containing bystander base edits. Subretinal injection of dual-adeno-associated viruses delivering our base editing strategy yielded up to 44% Rho correction in homozygous Rho-E150K mice. Injection at postnatal day 15, but not later time points, restored rhodopsin expression, partially rescued retinal function, and partially preserved retinal structure. These findings demonstrate that in vivo base editing can restore the function of mutated structural and functional proteins in animal models of disease, including rhodopsin-associated RP and suggest that the timing of gene-editing is a crucial determinant of successful treatment outcomes for degenerative genetic diseases.
Keywords: base editing; prime editing; retinitis pigmentosa; rhodopsin.
Conflict of interest statement
Competing interests statement:K.P. is a consultant for Polgenix Inc. and serves on the Scientific Advisory Board at Hyperion Eye Ltd. D.R.L. is a consultant and/or equity owner for Prime Medicine, Beam Therapeutics, Pairwise Plants, Chroma Medicine, and Nvelop Therapeutics, companies that use or deliver genome-editing or epigenome-engineering agents. One reviewer, A.V.C. is listed on a patent that is potentially competing to the work described in this paper. All other authors have declared that no conflict of interest exists.
Figures


References
-
- Palczewski K., et al. , Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 (2000). - PubMed
-
- Ripps H., The color purple: Milestones in photochemistry. FASEB J. 22, 4038–4043 (2008). - PubMed
-
- Böll F., Zur Anatomie und Physiologie der Retina. Monatsber Akad Wiss Berlin 783–788 (1876).
MeSH terms
Substances
Grants and funding
- UG3 AI150551/AI/NIAID NIH HHS/United States
- F30 EY033642/EY/NEI NIH HHS/United States
- F30EY029136 T32GM007250 T32AR080622/HHS | NIH (NIH)
- F30 EY029136/EY/NEI NIH HHS/United States
- RM1 HG009490/HG/NHGRI NIH HHS/United States
- UG3AI150551 U01AI142756 R35GM118062 RM1HG009490/HHS | NIH (NIH)
- R35 GM118062/GM/NIGMS NIH HHS/United States
- R00HL163805/HHS | NIH (NIH)
- U01 AI142756/AI/NIAID NIH HHS/United States
- R01 EY034501/EY/NEI NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
- R01 EY009339/EY/NEI NIH HHS/United States
- P30 EY034070/EY/NEI NIH HHS/United States
- T32 GM008620/GM/NIGMS NIH HHS/United States
- R00 HL163805/HL/NHLBI NIH HHS/United States
- R01EY009339 R01EY034501 P30EY034070/HHS | NIH (NIH)
- T32 AR080622/AR/NIAMS NIH HHS/United States
- T32GM008620 F30EY033642/HHS | NIH (NIH)

