Metabolic scaling, energy allocation tradeoffs, and the evolution of humans' unique metabolism
- PMID: 39556743
- PMCID: PMC11621513
- DOI: 10.1073/pnas.2409674121
Metabolic scaling, energy allocation tradeoffs, and the evolution of humans' unique metabolism
Abstract
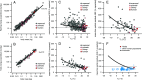
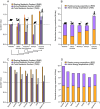
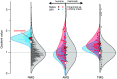
All organisms use limited energy to grow, survive, and reproduce, necessitating energy allocation tradeoffs, but there is debate over how selection impacted metabolic budgets and tradeoffs in primates, including humans. Here, we develop a method to compare metabolic rates as quotients of observed relative to expected values for mammals corrected for size, body composition, environmental temperature, and phylogenetic relatedness. Contrary to previous analyses, these quotients reveal that nonhuman primates have total metabolic rates expected for similar-sized mammals in similar environments. In addition, data from several small-scale societies show that humans evolved exceptionally high resting, activity, and total metabolic rates apparently by overcoming tradeoffs between resting and active energy expenditures that constrain other primates. Enhanced metabolic rates help humans fuel expanded brains, faster reproductive rates, extended longevity, and high percentage of body fat.
Keywords: evolution; human; metabolism; primate; tradeoffs.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

References
-
- Kaplan H., Hill K., Lancaster J., Hurtado A. M., A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. 9, 156–185 (2000).
-
- Wrangham R. W., Jones J. H., Laden G., Pilbeam D., Conklin-Brittain N., The raw and the stolen: Cooking and the ecology of human origins. Curr. Anthropol. 40, 567–594 (1999). - PubMed
-
- Kraft T. S., et al. , The energetics of uniquely human subsistence strategies. Science 374, eabf0130 (2022). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

