Treatment Intensification With Either Fludarabine, AraC, G-CSF and Idarubicin, or Cladribine Plus Daunorubicin and AraC on the Basis of Residual Disease Status in Older Patients With AML: Results From the NCRI AML18 Trial
- PMID: 39556780
- PMCID: PMC11825491
- DOI: 10.1200/JCO.24.00259
Treatment Intensification With Either Fludarabine, AraC, G-CSF and Idarubicin, or Cladribine Plus Daunorubicin and AraC on the Basis of Residual Disease Status in Older Patients With AML: Results From the NCRI AML18 Trial
Erratum in
-
Erratum: Treatment Intensification With Either Fludarabine, AraC, G-CSF and Idarubicin, or Cladribine Plus Daunorubicin and AraC on the Basis of Residual Disease Status in Older Patients With AML: Results From the NCRI AML18 Trial.J Clin Oncol. 2025 Jan 10;43(2):239. doi: 10.1200/JCO-24-02577. Epub 2024 Dec 3. J Clin Oncol. 2025. PMID: 39626163 Free PMC article. No abstract available.
Abstract
Purpose: To evaluate the survival benefit of chemotherapy intensification in older patients with AML who have not achieved a measurable residual disease (MRD)-negative remission.
Methods: Five hundred twenty-three patients with AML (median age, 67 years; range, 51-79) without a flow cytometric MRD-negative remission response after a first course of daunorubicin and AraC (DA; including 165 not in remission) were randomly assigned between up to two further courses of DA or intensified chemotherapy-either fludarabine, cytarabine, granulocyte colony-stimulating factor and idarubicin (FLAG-Ida) or DA with cladribine (DAC).
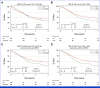
Results: Overall survival (OS) was not improved in the intensification arms (DAC v DA: hazard ratio [HR], 0.74 [95% CI, 0.55 to 1.01]; P = .054; FLAG-Ida v DA: HR, 0.86 [95% CI, 0.66 to 1.12]; P = .270); OS at 3 years was 34%, 46%, and 42% for DA, DAC, and FLAG-Ida, respectively. Early deaths and other adverse events were more frequent with FLAG-Ida (9% day 60 deaths v 4% after DA or DAC; P = .032). Of patients entering random assignment, 131 had MRD unknown status. In this subgroup of patients lacking evidence of residual leukemia by flow cytometry, there was no detectable survival advantage from intensification. A planned sensitivity analysis excluding these patients demonstrated a survival benefit for both DAC (HR, 0.66 [95% CI, 0.46 to 0.93]; P = .018) and FLAG-Ida (HR, 0.72 [95% CI, 0.53 to 0.98]; P = .035); OS at 3 years was 30%, 46%, and 46% for DA, DAC, and FLAG-Ida, respectively. There was a concordant reduction in relapse (DAC v DA: HR, 0.66 [95% CI, 0.45 to 0.98]; P = .039; FLAG-Ida v DA: HR, 0.70 [95% CI, 0.49 to 0.99]; P = .042). DAC benefit was maintained when survival was censored for transplant (P = .042).
Conclusion: In this study of older patients with AML considered fit and with evidence of residual disease after first induction, chemotherapy intensification improved survival. DAC intensification was better tolerated than FLAG-Ida.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures



References
-
- Freeman SD, Virgo P, Couzens S, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31:4123–4131. - PubMed
-
- Dohner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. - PubMed
-
- Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368. - PubMed
-
- Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J Clin Oncol. 2012;30:2441–2448. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

