Efficacy and acceptability of lurasidone for bipolar depression: a systematic review and dose-response meta-analysis
- PMID: 39557452
- PMCID: PMC11574478
- DOI: 10.1136/bmjment-2024-301165
Efficacy and acceptability of lurasidone for bipolar depression: a systematic review and dose-response meta-analysis
Abstract
Question: The optimal dose of lurasidone for bipolar depression is unclear. This study examined its dose-response relationship for efficacy, acceptability, and metabolic/endocrine profiles.
Study selection and analysis: Five databases and grey literature published until 1 August 2024, were systematically reviewed. The outcomes included efficacy (changes in depression, anxiety, clinical global impression, disability and quality of life), acceptability (dropout, manic switch, suicidality and side effects) and metabolic/endocrine profiles (changes in body weight, glucose, lipid and prolactin levels). Effect sizes were calculated using a one-step dose-response meta-analysis, expressed as standardised mean differences (SMDs), risk ratios (RRs) and mean differences (MDs) with 95% CIs.
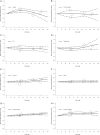
Findings: Five randomised clinical trials (2032 patients, mean treatment duration 6 weeks) indicated that the optimal therapeutic dose of lurasidone (40-60 mg) improved depression (50 mg: SMD -0.60 (95% CI -0.30, -0.89)), anxiety (50 mg: -0.32 (95% CI -0.21, -0.42)), clinical global impression (50 mg: -0.67 (95% CI -0.30, -1.03)) and disability (50 mg: -0.38 (95% CI -0.08, -0.69)). Side effects increased with higher doses (50 mg: RR 1.15 (95% CI 1.05, 1.25); 100 mg: 1.18 (95% CI 1.02, 1.36)), but dropout, manic switch and suicidality did not show a dose-effect relationship. Weight increased at doses<60 mg (40 mg: MD 0.38 (95% CI 0.16, 0.60) kg), while blood glucose levels rose at doses>70 mg (100 mg: 3.16 (95% CI 0.76, 5.57) mg/dL). Prolactin levels increased in both males (50 mg: 3.21 (95% CI 1.59, 4.84) ng/mL; 100 mg: 5.61 (95% CI 2.42, 8.81)) and females (50 mg: 6.64 (95% CI 3.50, 9.78); 100 mg: 5.33 (95% CI 0.67, 10.00)).
Conclusions: A daily dose of 40-60 mg of lurasidone is a reasonable choice for bipolar depression treatment.
Trial registration number: INPLASY202430069.
Keywords: Depression; Depression & mood disorders; PSYCHIATRY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: EV has received grants and served as a consultant, advisor, or CME speaker for the following entities: AB-Biotics, Abbott, AbbVie, Angelini, Biogen, Biohaven, Boehringer-Ingelheim, Bristol-Myers Squibb, Celon, Compass, Dainippon Sumitomo Pharma, Farmindustria, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith-Kline, HMNC, Idorsia, Janssen, Johnson & Johnson, Lundbeck, Medincell, Merck, Novartis, Orion, Otsuka, Pfizer, Roche, Rovi, Sanofi-Aventis, Sunovion, Takeda, Teva, Viatris, the Brain and Behaviour Foundation, the Spanish Ministry of Science and Innovation (CIBERSAM), the EU Horizon 2020, and the Stanley Medical Research Institute. MS received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck, Otsuka. ECCL reports research funding from Amgen, Pfizer, Novartis, Sanofi, Takeda, Roche, IQVIA, Clarivate, Novartis, Adelphi, the Taiwan National Science and Technology Council (NSTC 113-2628-B-006-009-) and the Taiwan National Health Research Institutes, outside the submitted work. Other authors declare no financial interests or potential conflicts of interest regarding the authorship and publication of this article.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
