Adoptive transfer of membrane-restricted IL-12-TCR T cells promotes antigen spreading and elimination of antigen-negative tumor variants
- PMID: 39557544
- PMCID: PMC11574437
- DOI: 10.1136/jitc-2024-009868
Adoptive transfer of membrane-restricted IL-12-TCR T cells promotes antigen spreading and elimination of antigen-negative tumor variants
Abstract
Background: Adoptive T-cell therapy has demonstrated clinical activity in B-cell malignancies, offering hope for its application to a broad spectrum of cancers. However, a significant portion of patients with solid tumors experience primary or secondary resistance to this treatment modality. Target antigen loss resulting either from non-uniform antigen expression or defects in antigen processing and presentation machinery is one well-characterized resistance mechanism. Constitutively expressed membrane-anchored interleukin-12 (caIL-12) has demonstrated enhanced antitumor activity and low systemic exposure in multiple preclinical adoptive T-cell treatment models with homogeneous tumor antigen expression. In this study, we assess the therapeutic impact of caIL-12 on target antigen-negative variants in syngeneic mouse models.
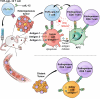
Methods: Target antigen-positive tumors were generated by transducing B16F10 melanoma cells (B16) or Lewis Lung Carcinoma cells (LLC) with a construct expressing the OVA antigen, SIINFEKL, tagged to ubiquitin (B16-U-OVA, LLC-U-OVA), while B16 or LLC tumors served as antigen-negative variants. C57BL/6J mice were subcutaneously injected with heterogeneous tumors composed of 80% B16-U-OVA and 20% B16. Bilateral tumors were established by injecting the left flank with B16-U-OVA or LLC-U-OVA tumors and the right flank injected with B16 or LLC tumors. The tumor-bearing mice then underwent 5.5 Gy total body irradiation, followed by adoptive transfer of OT-I TCR-T cells engineered with or without caIL-12.
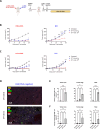
Results: TCR-T cells (OT-I) delivered caIL-12 to the B16-U-OVA tumor sites and induced robust tumor control and survival benefits in mice bearing a heterogeneous tumor with OVA-negative variants. caIL-12 exerted its effect on OVA-negative B16 variants primarily by priming and activating endogenous antitumor CD8 T cells via antigen spreading. In addition, antigen spreading induced by OT-I-caIL-12 resulted in controlling OVA-negative tumors implanted at distant sites. This therapeutic effect required antigen-specific TCR-T cells and caIL-12 to colocalize at the tumor site, along with endogenous CD8 T cells capable of recognizing shared tumor antigens.
Conclusion: Expression of caIL-12 by tumor-targeting T cells demonstrated therapeutic effect against target-antigen-negative tumor variants, primarily through the induction of antigen spreading. These findings highlight the potential of caIL-12 to address challenges of antigen escape and tumor heterogeneity that may limit the efficacy of T-cell therapy against solid tumors.
Keywords: Adoptive cell therapy - ACT; Cytokine; Solid tumor; T cell Receptor - TCR; Tumor infiltrating lymphocyte - TIL.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LZ is an inventor on NIH patent of inducible IL-12. CSH owns patents and royalties from multiple inventions in the field of immunotherapy and cellular therapy at the NIH and at Rutgers Cancer Institute, serves as a consultant or advisory board for Capstan Therapeutics, Neogene Therapeutics, Vir Biotechnology, sponsored research from Neogene Therapeutics, Iovance Biotherapeutics, equity and company officer for Scarlet TCR. The remaining authors declare no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
