Human iPSC-derived microglia sense and dampen hyperexcitability of cortical neurons carrying the epilepsy-associated SCN2A-L1342P mutation
- PMID: 39557580
- PMCID: PMC11735681
- DOI: 10.1523/JNEUROSCI.2027-23.2024
Human iPSC-derived microglia sense and dampen hyperexcitability of cortical neurons carrying the epilepsy-associated SCN2A-L1342P mutation
Abstract
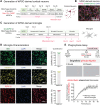
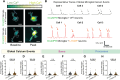
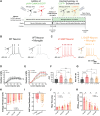
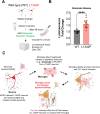
Neuronal hyperexcitability is a hallmark of epilepsy. It has been recently shown in rodent models of seizures that microglia, the brain's resident immune cells, can respond to and modulate neuronal excitability. However, how human microglia interact with human neurons to regulate hyperexcitability mediated by an epilepsy-causing genetic mutation found in patients is unknown. The SCN2A gene is responsible for encoding the voltage-gated sodium channel Nav1.2, one of the leading contributors to monogenic epilepsies. Previously, we demonstrated that the recurring Nav1.2-L1342P mutation leads to hyperexcitability in a male donor (KOLF2.1) hiPSC-derived cortical neuron model. Microglia originate from a different lineage (yolk sac) and are not naturally present in hiPSCs-derived neuronal cultures. To study how microglia respond to neurons carrying a disease-causing mutation and influence neuronal excitability, we established a co-culture model comprising hiPSC-derived neurons and microglia. We found that microglia display increased branch length and enhanced process-specific calcium signal when co-cultured with Nav1.2-L1342P neurons. Moreover, the presence of microglia significantly lowered the repetitive action potential firing and current density of sodium channels in neurons carrying the mutation. Additionally, we showed that co-culturing with microglia led to a reduction in sodium channel expression within the axon initial segment of Nav1.2-L1342P neurons. Furthermore, we demonstrated that Nav1.2-L1342P neurons release a higher amount of glutamate compared to control neurons. Our work thus reveals a critical role of human iPSCs-derived microglia in sensing and dampening hyperexcitability mediated by an epilepsy-causing mutation.Significance Statement Seizure studies in mouse models have highlighted the role of microglia in modulating neuronal activity, particularly in the promotion or suppression of seizures. However, a gap persists in comprehending the influence of human microglia on intrinsically hyperexcitable neurons carrying epilepsy-associated pathogenic mutations. This research addresses this gap by investigating human microglia and their impact on neuronal functions. Our findings demonstrate that microglia exhibit dynamic morphological alterations and calcium fluctuations in the presence of neurons carrying an epilepsy-associated SCN2A mutation. Furthermore, microglia suppressed the excitability of hyperexcitable neurons, suggesting a potential beneficial role. This study underscores the role of microglia in the regulation of abnormal neuronal activity, providing insights into therapeutic strategies for neurological conditions associated with hyperexcitability.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests. ChatGPT was used to improve the readability and language of this work.
Figures



Update of
-
Human iPSC-derived microglia sense and dampen hyperexcitability of cortical neurons carrying the epilepsy-associated SCN2A-L1342P mutation.bioRxiv [Preprint]. 2023 Oct 31:2023.10.26.563426. doi: 10.1101/2023.10.26.563426. bioRxiv. 2023. Update in: J Neurosci. 2024 Nov 18:e2027232024. doi: 10.1523/JNEUROSCI.2027-23.2024. PMID: 37961213 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
