Challenging dynamic cerebral autoregulation across the physiological CO2 spectrum: Influence of biological sex and cardiac cycle
- PMID: 39557629
- PMCID: PMC11689124
- DOI: 10.1113/EP092245
Challenging dynamic cerebral autoregulation across the physiological CO2 spectrum: Influence of biological sex and cardiac cycle
Abstract
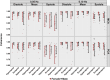
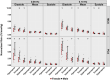
This study applied alterations in partial pressure of end-tidal carbon dioxide ( ) to challenge dynamic cerebral autoregulation (dCA) responses across the cardiac cycle in both biological sexes. A total of 20 participants (10 females and 10 males; aged 19-34 years) performed 4-min bouts of repeated squat-stand manoeuvres (SSMs) at 0.05 and 0.10 Hz (randomized orders) with clamped at ∼40 mmHg. The protocol was repeated for hypercapnic (∼55 mmHg) and hypocapnic (∼20 mmHg) conditions. Middle cerebral artery (MCA) and posterior cerebral artery (PCA) were insonated via transcranial Doppler ultrasound. Dynamic end-tidal forcing clamped , and finger photoplethysmography quantified beat-to-beat changes in blood pressure. Linear regressions were performed for transfer function analysis metrics including power spectrum densities, coherence, phase, gain and normalized gain (nGain) with adjustment for sex. During hypercapnic conditions, phase metrics were reduced from eucapnic levels (all P < 0.009), while phase increased during the hypocapnic stage during both 0.05 and 0.10 Hz SSMs (all P < 0.037). Sex differences were present with females displaying greater gain and nGain systole metrics during 0.10 Hz SSMs (all P < 0.041). Across stages, females displayed reduced buffering against systolic aspects of the cardiac cycle and augmented gain. Sex-related variances in dCA could explain sex differences in the occurrence of clinical conditions such as orthostatic intolerance and stroke, though the effect of fluctuating sex hormones and contraceptive use on dCA metrics is not yet understood.
Keywords: dynamic end‐tidal forcing; eucapnia; hypercapnia; hypocapnia; sex differences; squat–stand manoeuvres.
© 2024 The Author(s). Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures




References
-
- Aaslid, R. , Lindegaard, K. F. , Sorteberg, W. , & Nornes, H. (1989). Cerebral autoregulation dynamics in humans. Stroke, 20(1), 45–52. - PubMed
-
- Abdu, H. , & Seyoum, G. (2022). Sex differences in stroke risk factors, clinical profiles, and in‐hospital outcomes among stroke patients admitted to the medical ward of dessie comprehensive specialized hospital, Northeast Ethiopia. Degenerative Neurological and Neuromuscular Disease, 12, 133–144. - PMC - PubMed
-
- Ainslie, P. N. , Barach, A. , Murrell, C. , Hamlin, M. , Hellemans, J. , & Ogoh, S. (2007). Alterations in cerebral autoregulation and cerebral blood flow velocity during acute hypoxia: Rest and exercise. American Journal of Physiology‐Heart and Circulatory Physiology, 292(2), H976–H983. - PubMed
-
- Ainslie, P. N. , Cotter, J. D. , George, K. P. , Lucas, S. , Murrell, C. , Shave, R. , Thomas, K. N. , Williams, M. J. A. , & Atkinson, G. (2008). Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. The Journal of Physiology, 586(16), 4005–4010. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
