Feedback driven autonomous cycles of assembly and disassembly from minimal building blocks
- PMID: 39557837
- PMCID: PMC11574191
- DOI: 10.1038/s41467-024-54197-y
Feedback driven autonomous cycles of assembly and disassembly from minimal building blocks
Abstract
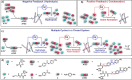
The construction of complex systems by simple chemicals that can display emergent network dynamics might contribute to our understanding of complex behavior from simple organic reactions. Here we design single amino acid/dipeptide-based systems that exhibit multiple periodic changes of (dis)assembly under non-equilibrium conditions in closed system, importantly in the absence of evolved biocatalysts. The two-component based building block exploits pH driven non-covalent assembly and time-delayed accelerated catalysis from self-assembled state to install orthogonal feedback loops with a single batch of reactants. Mathematical modelling of the reaction network establishes that the oscillations are transient for this network structure and helps to predict the relative contribution of the feedback loop to the ability of the system to exhibit such transient oscillation. Such autonomous systems with purely synthetic molecules are the starting point that can enable the design of active materials with emergent properties.
© 2024. The Author(s).
Conflict of interest statement
Figures





References
-
- Grzybowski, B. A. & Huck, W. T. S. The nanotechnology of life-inspired systems. Nat. Nanotechnol.11, 585–592 (2016). - PubMed
-
- Ragazzon, G. & Prins, L. J. Energy consumption in chemical fuel-driven self-assembly. Nat. Nanotechnol.13, 882–889 (2018). - PubMed
-
- He, X. et al. Synthetic homeostatic materials with chemo-mechano-chemical self-regulation. Nature487, 214–218 (2012). - PubMed
-
- Bal, S., Ghosh, C., Ghosh, T., Vijayaraghavan, R. K. & Das, D. Non-equilibrium polymerization of cross-β amyloid peptides for temporal control of electronic properties. Angew. Chem. Int. Ed.59, 13506–13510 (2020). - PubMed
-
- Ashkenasy, G., Hermans, T. M., Otto, S. & Taylor, A. F. Systems chemistry. Chem. Soc. Rev.46, 2543–2554 (2017). - PubMed
LinkOut - more resources
Full Text Sources

